Abstract
Background
The objective of this study was to evaluate the efficacy and safety of induction chemotherapy (ICT), GOFL (gemcitabine, oxaliplatin plus fluorouracil (5-FU)/leucovorin) versus modified FOLFIRINOX (irinotecan, oxaliplatin plus 5-FU/leucovorin), followed by concurrent chemoradiotherapy (CCRT) in locally advanced pancreatic adenocarcinoma (LAPC).
Methods
Chemo-naive patients with measurable LAPC were eligible and randomly assigned to receive biweekly ICT with either mFOLFIRINOX or GOFL for 3 months. Patients without systemic progression would have 5-FU- or gemcitabine-based CCRT (5040 cGy/28 fractions) and were then subjected to surgery or continuation of chemotherapy until treatment failure. The primary endpoint was 9-month progression-free survival (PFS) rate.
Results
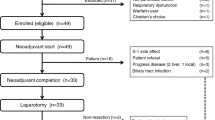
Between July 2013 and January 2019, 55 patients were enrolled. After ICT, 21 (77.8%) of 27 patients who received mFOLFIRINOX and 17 (60.7%) of 28 patients who received GOFL completed CCRT. Of them, one and five had per-protocol R0/R1 resection. On intent-to-treat analysis, the 9-month PFS rate, median PFS and overall survival in mFOLFIRINOX and GOFL arms were 30.5% versus 35.9%, 6.6 (95% confidence interval: 5.9–12.5) versus 7.6 months (3.9–12.3) and 19.6 (13.4–22.9) versus 17.9 months (13.4–23.9), respectively. Grade 3–4 neutropenia and diarrhoea during induction mFOLFIRINOX and GOFL were 37.0% versus 21.4% and 14.8% versus 3.6%, respectively.
Conclusion
Induction GOFL and mFOLFIRINOX followed by CCRT provided similar clinical outcomes in LAPC patients.
Clinicaltrial.gov identifier
NCT01867892.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data generated and analysed during this study are included in this article and its Supplementary information files.
References
Sultana A, Tudur Smith C, Cunningham D, Starling N, Tait D, Neoptolemos JP, et al. Systematic review, including meta-analyses, on the management of locally advanced pancreatic cancer using radiation/combined modality therapy. Br J Cancer. 2007;96:1183–90.
Seufferlein T, Hammel P, Delpero JR, Macarulla T, Pfeiffer P, Prager GW, et al. Optimizing the management of locally advanced pancreatic cancer with a focus on induction chemotherapy: Expert opinion based on a review of current evidence. Cancer Treat Rev. 2019;77:1–10.
Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, et al. Randomized phase III multi-institutional study of TNFerade biologic with flurorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol. 2013;31:886–94.
Chang JS, Chiu YF, Yu JC, Chen LT, Ch’ang HJ. The role of consolidation chemoradiotherapy in locally advanced pancreatic cancer receiving chemotherapy: an updated systemic review and meta-analysis. Cancer Res Treat. 2018;50:562–74.
Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients With locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 randomized clinical trial. JAMA. 2016;315:1844–53.
Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14:317–26.
Ch’ang HJ, Wang CC, Cheng AL, Hsu C, Lu YS, Chang MC, et al. Phase I study of biweekly gemcitabine followed by oxaliplatin and simplified 48-h infusion of fluorouracil/leucovorin for advanced pancreatic cancer. J Gastroenterol Hepatol. 2006;21:874–9.
Ch’ang HJ, Huang CL, Wang HP, Shiah HS, Chang MC, Jan CM, et al. Phase II study of biweekly gemcitabine followed by oxaliplatin and simplified 48-h infusion of 5-fluorouracil/leucovorin (GOFL) in advanced pancreatic cancer. Cancer Chemother Pharmacol. 2009;64:1173–9.
Ch’ang HJ, Lin YL, Wang HP, Chiu YF, Chang MC, Hsu CH, et al. Induction chemotherapy with gemcitabine, oxaliplatin, and 5-fluorouracil/leucovorin followed by concomitant chemoradiotherapy in patients with locally advanced pancreatic cancer: a Taiwan Cooperative Oncology Group phase II study. Int J Radiat Oncol Biol Phys. 2011;81:e749–757.
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Balaban EP, Mangu PB, Khorana AA. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2654–68.
Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma, version 2021.2. J Natl Compr Cancer Netw. 2021;19:439–57.
Okusaka T, Nakamura M, Yoshida M, Kitano M, Uesaka K, Ito Y, et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: a synopsis. Pancreas. 2020;49:326–35.
Reames BN, Blair AB, Krell RW, Groot VP, Gemenetzis G, Padussis JC, et al. Management of locally advanced pancreatic cancer” results of an international survey of current practice. Ann Surg. 2021;273:1173–81.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
National Institutes of Health. National Cancer Institute: Common Terminology Criteria for Adverse Events version 4.03. 2009. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_4.03.xlsx Accessed 11 Oct 2021.
Conroy T, Paillot B, Fran.ois E, Bugat R, Jacob JH, Stein U, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer—a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. 2005;23:1228–36.
Liu PY, Dahlberg S, Crowley J. Selection designs for pilot studies based on survival. Biometrics. 1993;49:391–8.
Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–8.
Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–57.
Chao YJ, Sy ED, Hsu HP, Shan YS. Predictors for resectability and survival in locally advanced pancreatic cancer after gemcitabine-based neoadjuvant therapy. BMC Surg. 2014;14:72.
Kunzmann V, Siveke JT, Algu¨l H, Goekkurt E, Siegler G, Martens U, et al. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicenter randomised phase II trial. Lancet Gastroenterol Hepatol. 2021;6:128–38.
Ozaka M, Ishii H, Sato T, Ueno M, Ikeda M, Uesugi K, et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2018;81:1017–23.
Conroy T, Hammel P, Hebbar M, Abdelghani MB, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl J Med. 2018;379:2395–406.
Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:737–43.
Ozaka M, Ueno M, Ishii H, Mizusawa J, Katayama H, Kataoka T, et al. Randomized phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel combination therapy for locally advanced pancreatic cancer (JCOG1407). J Clin Oncol. 2021;39 Suppl 15;abstract 4017.
Li CP, Chao Y, Chi KH, Chan WK, Teng HC, Lee RC, et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys. 2003;57:98–104.
Ioka T, Furuse J, Fukutomi A, Mizusawa J, Nakamura S, Hiraok N, et al. Randomized phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncology Group trial, JCOG1106. Jpn J Clin Oncol. 2021;51:235–43.
Ikeda M, Ioka T, Ito Y, Yonemoto N, Nagase M, Yamao K, et al. A multicenter phase II trial of S-1 with concurrent radiation therapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:163–9.
Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, Mozo JLM, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicenter, open-label phase 2 study. Lancet. Gastroenterol Hepatol. 2020;5:285–94.
Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with Losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1020–7.
Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T, Omuro Y, et al. Phase I/II study of nab-paclitaxel plus gemcitabine for chemonaïve Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77:595–603.
Chiang NJ, Tsai KK, Hsiao CF, Yang SH, Hsiao HH, Shen WC, et al. A multicenter, phase I/II trial of biweekly S-1, leucovorin, oxaliplatin and gemcitabine in metastatic pancreatic adenocarcinoma-TCOG T1211 study. Eur J Cancer. 2020;124:123–30.
Chiang NJ, Shan YS, Bai LY, Li CP, Chen JS, Yang SH, et al. TCOG T5217 trial: a phase II randomized study of SLOG vs modified FOLFIRINOX as first-line treatment in locally advanced or metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2021;39 Suppl 15;abstract 4143.
Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020;38:3217–30.
Chen CY, Liang SH, Su YY, Chiang NJ, Wang HC, Chiu CF, et al. Modified gemcitabine, S-1, and leucovorin combination for patients with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma: a multi-center retrospective study in Taiwan. PLoS ONE. 2020;15:e0244487.
Lan KK, Wittes J. The B-value: a tool for monitoring data. Biometrics. 1988;44:579–85.
Shan YS, Chen LT, Wu JS, Chang YF, Lee CT, Wu CH, et al. Validation of genome-wide association study-identified single nucleotide polymorphisms in a case-control study of pancreatic cancer from Taiwan. J Biomed Sci. 2020;27:69.
Acknowledgements
We thank all the hospitals for enrolling patients in this trial including National Cheng Kung University Hospital, Tainan (to Y-SS, N-JC); Veterans General Hospital, Taipei City (to C-PL); Mackay Memorial Hospital, Taipei (to JL); National Taiwan University Hospital, Taipei City (to S-HY); Veterans General Hospital, Kaohsiung City (to S-JL, Ming-Sun Yu), Tri-Service General Hospital, Taipei (to P-YC, J-HC), Linkou Chang Gung Memorial Hospital, Taoyuan (to J-SC) and Taipei Medical University Hospital, Taipei City (to Her-Shyong Shiah). We are grateful for the participation of all the patients and their families and to make this study possible, and also for the help of research nurses and statisticians from the Taiwan Cooperative Oncology Group. In particular, we would like to thank Ms. Shu-Chuan Lai for her tremendous efforts in the comprehensive statistical analyses of this trial. Oxaliplatin (Oxalip®) and irinotecan (Irino®) were kindly sponsored by TTY Biopharm Company Limited, Taipei, Taiwan.
Funding
This study was conducted by the Taiwan Cooperative Oncology Group with funding (number CA-101–108-PP 25) from National Health Research Institutes, Ministry of Health and Welfare, Executive Yuan, Taiwan.
Author information
Authors and Affiliations
Contributions
H-JC, Y-SS, L-TC and Y-YS: conceptualisation, funding acquisition and writing—review & editing; Y-YS, Y-SS, C-PL, S-HY, JL, S-JL, P-YC, N-JC, L-TC and H-JC: data curation; Y-YS, H-JC, Y-FC, L-TC: formal analysis, methodology and writing—original draft; H-JC: project administration; Y-FC, Y-YS: software; Y-SS and L-TC: supervision; Y-YS, Y-SS, C-PL, S-HY, JL, S-HY, S-HL, P-YC, N-JC and L-TC: validation.
Corresponding authors
Ethics declarations
Competing interests
L-TC reports research funding from Novartis, Merck, Serono, TTY, Polaris, SyncorePharm, Pfizer, BMS; honoraria from ONO, Eli Lilly, MSD, PharmaEngine, TTY, SyncorePharm, Novartis, Astra Zeneca, Ipsen; patents and royalties from ENO-1mAb/HuniLife; membership on Board of Directors of ScinoPharm, Taiwan, Ltd. and on Scientific Advisory Committee of PharmaEngine. All the remaining authors declare no competing interests.
Ethics approval and consent to participate
The study protocol and informed consent forms were approved by the Research Ethics Committee, National Health Research Institutes, Taiwan (reference number: EC1010805) and the Institutional Review Board of each participating hospital. All subjects provided written informed consent prior to participating in the study. The study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Su, YY., Chiu, YF., Li, CP. et al. A phase II randomised trial of induction chemotherapy followed by concurrent chemoradiotherapy in locally advanced pancreatic cancer: the Taiwan Cooperative Oncology Group T2212 study. Br J Cancer 126, 1018–1026 (2022). https://doi.org/10.1038/s41416-021-01649-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01649-7
This article is cited by
-
Germline mutations of homologous recombination genes and clinical outcomes in pancreatic cancer: a multicenter study in Taiwan
Journal of Biomedical Science (2024)
-
Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer
Nature Reviews Gastroenterology & Hepatology (2024)
-
Proton radiotherapy as a treatment strategy to increase survival in locally advanced pancreatic cancer in the body and tail: a retrospective study
Radiation Oncology (2023)