Abstract
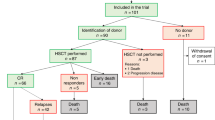
Data regarding the safety and efficacy of reduced-toxicity conditioning regimen (RTC) prior to allogeneic stem cell transplantation (allo-SCT) to treat hematological malignancies in pediatric patients are limited. This prospective multicenter, phase 2 trial investigated a RTC regimen based on the combination of intravenous busulfan (3.2 mg/kg/d x 4 days), fludarabine (30 mg/m2/d x 5 days) and antithymocyte globulin (Thymoglobulin®, Genzyme; 5 mg/kg total dose) with the aim of delivering high dose myeloablation that would allow optimal disease control while minimizing toxicity, in a subgroup of children at very high risk of non-relapse mortality (NRM). The primary endpoint was NRM at 1 year after allo-SCT. A total of 48 high risk patients were included (median age, 13 years; range, 3–24). At 1 year, the cumulative incidence of recurrence/disease progression and NRM were 33% and 8%, respectively. With a median follow-up of 23 months, the Kaplan-Meier estimates of overall survival (OS) and disease-free survival (DFS) at 1 year were 69% and 58%, respectively. We conclude that the RTC regimen used in this prospective trial is safe, with a < 10% NRM rate noted among high-risk children and adolescents, paving the way for larger phase 3 trials incorporating novel agents pre- and post-allo-SCT.
(ClinicalTrials.gov Identifier: NCT01572181)
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data are available upon request to ML or MM.
References
Mohty M, Labopin M, Volin L, Gratwohl A, Socié G, Esteve J, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: A retrospective study from the European Group for blood and marrow transplantation. Blood. 2010;116:4439–43. https://doi.org/10.1182/blood-2010-02-266551
Pulsipher MA, Levine JE, Hayashi RJ, Chan KW, Anderson P, Osunkwo I, et al. Safety and efficacy of allogeneic PBSC collection in normal pediatric donors: The pediatric blood and marrow transplant consortium experience (PBMTC) 1996-2003. Bone Marrow Transpl. 2005;35:361–7. https://doi.org/10.1038/sj.bmt.1704743
Pulsipher MA, Nagler A, Iannone R, Nelson RM. Weighing the risks of G-CSF administration, leukopheresis, and standard marrow harvest: Ethical and safety considerations for normal pediatric hematopoietic cell donors. Pediatr Blood Cancer. 2006;46:422–33. https://doi.org/10.1002/pbc.20708
Pulsipher MA, Boucher KM, Wall D, Frangoul H, Duval M, Goyal RK, et al. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood. 2009;114:1429–36. https://doi.org/10.1182/blood-2009-01-196303
Satwani P, Cooper N, Rao K, Veys P, Amrolia P. Reduced intensity conditioning and allogeneic stem cell transplantation in childhood malignant and nonmalignant diseases. Bone Marrow Transpl. 2008;41:173–82. https://doi.org/10.1038/sj.bmt.1705923
Blaise D, Vey N, Faucher C, Mohty M. Current status of reduced-intensity-conditioning allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2007;92:533–41. https://doi.org/10.3324/haematol.10867
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Rossoff J, Jacobsohn D, Kwon S, Kletzel M, Duerst RE, Tse WT, et al. Reduced-toxicity conditioning regimen with busulfan, fludarabine, rATG, and 400 cGy TBI in pediatric patients undergoing hematopoietic stem cell transplant for high-risk hematologic malignancies. Pediatr Blood Cancer. 2021;68:e29087 https://doi.org/10.1002/pbc.29087
Yaniv I, Krauss AC, Beohou E, Dalissier A, Corbacioglu S, Zecca M, et al. Second hematopoietic stem cell transplantation for post-transplantation relapsed acute leukemia in children: A retrospective EBMT-PDWP study. Biol Blood Marrow Transpl. 2018;24:1629–42. https://doi.org/10.1016/j.bbmt.2018.03.002
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. https://doi.org/10.1182/blood-2005-05-2004
Broglie L, Ruiz J, Jin Z, Kahn J, Bhatia M, George D, et al. Limitations of Applying the Hematopoietic Cell Transplantation Comorbidity Index in Pediatric Patients Receiving Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. Published online October 8, 2020. https://doi.org/10.1016/j.bbmt.2020.10.003
Friend BD, Tang K, Markovic D, Elashoff D, Moore TB, Schiller GJ. Identifying risk factors associated with worse outcomes in adolescents and young adults undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2019;66:e27940 https://doi.org/10.1002/pbc.27940
Peters C, Dalle JH, Locatelli F, Poetschger U, Sedlacek P, Buechner J, et al. Total body irradiation or chemotherapy conditioning in childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J Clin Oncol J Am Soc Clin Oncol. 2021;39:295–307. https://doi.org/10.1200/JCO.20.02529
Eapen M, Horowitz MM, Klein JP, Champlin RE, Loberitza FR Jr, Ringden O, et al. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: The Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol J Am Soc Clin Oncol. 2004;22:4872–80. https://doi.org/10.1200/JCO.2004.02.189
Lucchini G, Labopin M, Beohou E, Dalissier A, Dalle JH, Cornish J, et al. Impact of conditioning regimen on outcomes for children with acute myeloid leukemia undergoing transplantation in first complete remission. an analysis on behalf of the pediatric disease working party of the European group for blood and marrow transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2017;23:467–74. https://doi.org/10.1016/j.bbmt.2016.11.022
Sauer MG, Lang PJ, Albert MH, Bader P, Creutzig U, Eyrich M, et al. Hematopoietic stem cell transplantation for children with acute myeloid leukemia—results of the AML SCT-BFM 2007 trial. Leukemia. 2020;34:613–24. https://doi.org/10.1038/s41375-019-0584-8
Ishida H, Adachi S, Hasegawa D, Okamoto Y, Goto H, Inagaki J, et al. Comparison of a fludarabine and melphalan combination-based reduced toxicity conditioning with myeloablative conditioning by radiation and/or busulfan in acute myeloid leukemia in Japanese children and adolescents. Pediatr Blood Cancer. 2015;62:883–9. https://doi.org/10.1002/pbc.25389
Balduzzi A, Dalle JH, Wachowiak J, Yaniv I, Yesilipek A, Sedlacek P, et al. Transplantation in Children and Adolescents with Acute Lymphoblastic Leukemia from a Matched Donor versus an HLA-Identical Sibling: Is the Outcome Comparable? Results from the International BFM ALL SCT 2007 Study. Biol Blood Marrow Transpl. 2019;25:2197–210. https://doi.org/10.1016/j.bbmt.2019.07.011
Hirabayashi K, Nakazawa Y, Sakashita K, Kurata T, Saito S, Yoshikawa K, et al. Reduced-toxicity myeloablative conditioning consisting of 8-Gy total body irradiation, cyclophosphamide and fludarabine for pediatric hematological malignancies. Sci Rep. 2014;4:6942 https://doi.org/10.1038/srep06942
Ueda M, de Lima M, Caimi P, Tomlinson B, Little J, Creger R, et al. Concurrent blinatumomab and donor lymphocyte infusions for treatment of relapsed pre-B-cell ALL after allogeneic hematopoietic cell transplant. Bone Marrow Transpl. 2016;51:1253–5. https://doi.org/10.1038/bmt.2016.104
Zahler S, Bhatia M, Ricci A, Roy S, Morris E, Harrison L, et al. A Phase I Study of Reduced-Intensity Conditioning and Allogeneic Stem Cell Transplantation Followed by Dose Escalation of Targeted Consolidation Immunotherapy with Gemtuzumab Ozogamicin in Children and Adolescents with CD33+ Acute Myeloid Leukemia. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transpl. 2016;22:698–704. https://doi.org/10.1016/j.bbmt.2016.01.019
Acknowledgements
This trial was supported by the French national cancer institute (INCa) through a grant as part of the “Programme Hospitalier de Recherche Clinique” (PHRC-Cancer)
Author information
Authors and Affiliations
Contributions
MM, ML, and FR designed the research, collected and analyzed the data, and wrote the paper. All co-authors enrolled and treated patients, and participated in the manuscript. All co-authors approved the paper.
Corresponding author
Ethics declarations
Competing interests
MM has received lecture honoraria and research support from Pierre-Fabre Medicament, and Sanofi whose products are studied here. FR: none. ML: none. JHD: received honoraria and research financial support from Sanofi.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rialland, F., Grain, A., Labopin, M. et al. Reduced-toxicity myeloablative conditioning regimen using fludarabine and full doses of intravenous busulfan in pediatric patients not eligible for standard myeloablative conditioning regimens: Results of a multicenter prospective phase 2 trial. Bone Marrow Transplant 57, 1698–1703 (2022). https://doi.org/10.1038/s41409-022-01769-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01769-5