Abstract
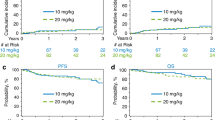
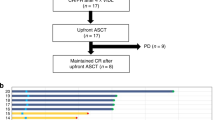
We analysed the therapeutic outcomes of all consecutive patients with primary central nervous system lymphoma (PCNSL) registered in the prospective French database for PCNSL and treated with intensive chemotherapy (IC) followed by autologous stem cell transplantation (IC-ASCT) between 2011 and November 2019 (271 patients recruited, 266 analysed). In addition, treatment-related complications of thiotepa-based IC-ASCT were analysed from the source files of 85 patients from 3 centers. Patients had received IC-ASCT either in first-line treatment (n = 147) or at relapse (n = 119). The median age at IC-ASCT was 57 years (range: 22–74). IC consisted of thiotepa-BCNU (n = 64), thiotepa-busulfan (n = 24), BCNU-etoposide-cytarabine-melphalan (BEAM, n = 36) and thiotepa-busulfan-cyclophosphamide (n = 142). In multivariate analysis, BEAM and ASCT beyond the first relapse were adverse prognostic factors for relapse risk. The risk of treatment-related mortality was higher for ASCT performed beyond the first relapse and seemed higher for thiotepa-busulfan-cyclophosphamide. Thiotepa-BCNU tends to result in a higher relapse rate than thiotepa-busulfan-cyclophosphamide and thiotepa-busulfan. This study confirms the role of IC-ASCT in first-line treatment and at first-relapse PCNSL (5-year overall survival rates of 80 and 50%, respectively). The benefit/risk ratio of thiotepa-busulfan/thiotepa-busulfan-cyclophosphamide-ASCT could be improved by considering ASCT earlier in the course of the disease and dose adjustment of the IC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Houillier C, Soussain C, Ghesquières H, Soubeyran P, Chinot O, Taillandier L, et al. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study. Neurology. 2020;94:e1027–e1039.
Omuro AMP, Ben-Porat LS, Panageas KS, Kim AK, Correa DD, Yahalom J, et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol. 2005;62:1595–600.
Doolittle ND, Korfel A, Lubow MA, Schorb E, Schlegel U, Rogowski S, et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology. 2013;81:84–92.
Schlegel U, Korfel A. Is whole-brain radiotherapy still a standard treatment for primary central nervous system lymphomas? Curr Opin Neurol. 2018;31:733–9.
Morris PG, Correa DD, Yahalom J, Raizer JJ, Schiff D, Grant B, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31:3971–9.
Correa DD, Braun E, Kryza-Lacombe M, Ho K-W, Reiner AS, Panageas KS, et al. Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J Neurooncol. 2019;144:553–62.
Kim P, Omuro A. Consolidation therapy in primary central nervous system lymphoma. Curr Treat Options Oncol. 2020;21:74.
Rubenstein JL, Hsi ED, Johnson JL, Jung S-H, Nakashima MO, Grant B, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31:3061–8.
Houillier C, Taillandier L, Dureau S, Lamy T, Laadhari M, Chinot O, et al. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J Clin Oncol. 2019;JCO1800306.
Illerhaus G, Kasenda B, Ihorst G, Egerer G, Lamprecht M, Keller U, et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol. 2016;3:e388–97.
Ferreri AJM, Cwynarski K, Pulczynski E, Fox CP, Schorb E, La Rosée P, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4:e510–23.
Soussain C, Choquet S, Fourme E, Delgadillo D, Bouabdallah K, Ghesquières H, et al. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica. 2012;97:1751–6.
Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol. 2008;26:2512–8.
Langner-Lemercier S, Houillier C, Soussain C, Ghesquières H, Chinot O, Taillandier L, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18:1297–303.
Ferreri AJM, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood. 2016;127:1642–9.
Sanders S, Chua N, Larouche J-F, Owen C, Shafey M, Stewart DA. Outcomes of consecutively diagnosed primary central nervous system lymphoma patients using the alberta lymphoma clinical practice guideline incorporating thiotepa-busulfan conditioning for transplantation-eligible patients. Biol Blood Marrow Transpl. 2019;25:1505–10.
Schorb E, Kasenda B, Ihorst G, Scherer F, Wendler J, Isbell L, et al. High-dose chemotherapy and autologous stem cell transplant in elderly patients with primary CNS lymphoma: a pilot study. Blood Adv. 2020;4:3378–81.
Abrey LE, Batchelor TT, Ferreri AJM, Gospodarowicz M, Pulczynski EJ, Zucca E, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–43.
Bubalo J, Carpenter PA, Majhail N, Perales M-A, Marks DI, Shaughnessy P, et al. Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation practice guideline committee. Biol Blood Marrow Transpl. 2014;20:600–16.
Simon N, Coiteux V, Bruno B, Taque S, Charbonnier A, Souchet L. et al. Dose adaptation of thedrugs used for hematopoietic stem-cell transplantation in patients withcomorbidity: obesity, chronic renal disease or hepatopathy: guidelines from the francophone society of bone marrow transplantation and cellular therapy(SFGM-TC)]. Bull Cancer.2017;104:S99–105.
Schorb E, Fox CP, Fritsch K, Isbell L, Neubauer A, Tzalavras A, et al. High-dose thiotepa-based chemotherapy with autologous stem cell support in elderly patients with primary central nervous system lymphoma: a European retrospective study. Bone Marrow Transpl. 2017;52:1113–9.
Gandhi MK, Hoang T, Law SC, Brosda S, O’Rourke K, Tobin JWD, et al. EBV-associated primary CNS lymphoma occurring after immunosuppression is a distinct immunobiological entity. Blood. 2021;137:1468–77.
Alnahhas I, Jawish M, Alsawas M, Zukas A, Prokop L, Murad MH, et al. Autologous stem-cell transplantation for primary central nervous system lymphoma: systematic review and meta-analysis. Clin Lymphoma, Myeloma Leuk. 2019;19:e129–41.
Abrey LE, Moskowitz CH, Mason WP, Crump M, Stewart D, Forsyth P, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21:4151–6.
Brevet M, Garidi R, Gruson B, Royer B, Vaida I, Damaj G. First-line autologous stem cell transplantation in primary CNS lymphoma. Eur J Haematol. 2005;75:288–92.
Colombat P, Lemevel A, Bertrand P, Delwail V, Rachieru P, Brion A, et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transpl. 2006;38:417–20.
Khurana A, Micallef IN, LaPlant BR, Patrick O’Neill B, Habermann TM, Ansell SM, et al. Outcomes of autologous stem cell transplant consolidation in primary central nervous system lymphoma: A Mayo clinic experience. Biol Blood Marrow Transpl. 2020;26:2217–22.
Scordo M, Wang TP, Ahn KW, Chen Y, Ahmed S, Awan FT, et al. Outcomes associated with thiotepa-based conditioning in patients with primary central nervous system lymphoma after autologous hematopoietic cell transplant. JAMA Oncol. 2021;7:993–1003.
Scordo M, Bhatt V, Hsu M, Omuro AM, Matasar MJ, DeAngelis LM, et al. A comprehensive assessment of toxicities in patients with central nervous system lymphoma undergoing autologous stem cell transplantation using thiotepa, busulfan, and cyclophosphamide conditioning. Biol Blood Marrow Transpl. 2017;23:38–43.
Cote GM, Hochberg EP, Muzikansky A, Hochberg FH, Drappatz J, McAfee SL, et al. Autologous stem cell transplantation with thiotepa, busulfan, and cyclophosphamide (TBC) conditioning in patients with CNS involvement by non-Hodgkin lymphoma. Biol Blood Marrow Transpl. 2012;18:76–83.
DeFilipp Z, Li S, El-Jawahri A, Armand P, Nayak L, Wang N, et al. High-dose chemotherapy with thiotepa, busulfan, and cyclophosphamide and autologous stem cell transplantation for patients with primary central nervous system lymphoma in first complete remission. Cancer. 2017;123:3073–9.
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives—a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2015;50:781–9.
Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transpl. 2010;16:157–68.
Corbacioglu S, Jabbour EJ, Mohty M. Risk factors for development of and progression of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Biol Blood Marrow Transpl. 2019;25:1271–80.
Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444–57.
Alcantara M, Houillier C, Blonski M, Rubio M-T, Willems L, Waultier Rascalou A, et al. CAR-T cell therapy in primary central nervous system lymphoma (PCNSL): the clinical experience of the French LOC network. Blood. 2021;blood.2021012932.
Frigault MJ, Dietrich J, Martinez-Lage M, Leick M, Choi BD, DeFilipp Z, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134:860–6.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Acknowledgements
We thank the clinicians involved in the French National “Lymphome Oculo-Cérébral” LOC network, part of the network for rare cancers of the central nervous system, “RENOCLIP-LOC Network”, approved by the French National Institute of Cancer (INCa).
Author information
Authors and Affiliations
Consortia
Contributions
LS: conceived and designed the work that led to the submission, acquired data, and played an important role in interpreting the results, drafted and revised the manuscript, approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CS: conceived and designed the work that led to the submission, acquired data, and played an important role in interpreting the results, drafted and revised the manuscript, approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CH: Retrieved data from the database, approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MLT: performed the statistical analyses, revised the manuscript and approved the final version. LT: acquired data, approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SC: acquired data, approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. KA: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. HG: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. GD: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AS: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. KB: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. GA: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RG: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JC: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RH: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JPM: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LMF: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. OC: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. FP: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RB: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CMC: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. EG: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AC: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. OC: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LO: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. VDE: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JA: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. VR: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AWR: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LW: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. FM: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MF: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RU: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CT: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. FJ: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AT: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. DM: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. VT: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LN: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MLGT: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AP: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. PB: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CB: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SWM: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. QG: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. VD: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CC: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. WJ: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. KHX: acquired data and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Schenone, L., Houillier, C., Tanguy, M.L. et al. Intensive chemotherapy followed by autologous stem cell transplantation in primary central nervous system lymphomas (PCNSLs). Therapeutic outcomes in real life—experience of the French Network. Bone Marrow Transplant 57, 966–974 (2022). https://doi.org/10.1038/s41409-022-01648-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01648-z
This article is cited by
-
Antineoplastics
Reactions Weekly (2022)