Abstract
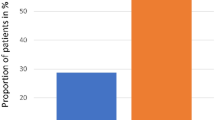
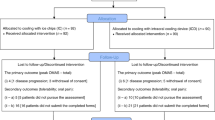
The conditioning therapy used in connection with haematopoietic stem cell transplantation (HSCT) can induce painful oral mucositis, which has negative impacts on patient quality of life and survival, as well as on health-care costs. While cooling of the oral mucosa (cryotherapy) is regarded as standard prophylaxis against oral mucositis, the long duration of the treatment affects compliance owing to side effects. In this prospective, randomised trial, 94 patients (62 males/32 females; median age 59 years, range 34–69) with a diagnosis of myeloma who were undergoing autologous HSCT were randomised 1:1 to receive cryotherapy for 7 h (N = 46) or 2 h (N = 48). Oral mucositis was evaluated prospectively. No significant difference was observed with respect to the proportion of patients who showed grades 3 and 4 toxicity according to the WHO scale (2.1 and 4.3% for 2 and 7 h, respectively; 95% CI −0.09 to 0.049; p = 0.98) as between the groups. Two hours of cryotherapy was as effective as 7 h in terms of protecting against severe oral mucositis in connection with autologous HSCT for myeloma. This trial is registered with ClinicalTrials.gov (NCT03704597).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Sureda A, Bader P, Cesaro S, Dreger P, Duarte RF, Dufour C, et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant. 2015;50:1037–56.
Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21:1863–9.
Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016;51:786–92.
D’Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT), CIBMTR Summary Slides, http://www.cibmtr.org. 2017.
Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol. 2001;19:2201–5.
Lilleby K, Garcia P, Gooley T, McDonnnell P, Taber R, Holmberg L, et al. A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37:1031–5.
Mahood DJ, Dose AM, Loprinzi CL, Veeder MH, Athmann LM, Therneau TM, et al. Inhibition of fluorouracil-induced stomatitis by oral cryotherapy. J Clin Oncol. 1991;9:449–52.
Bensinger W, Schubert M, Ang KK, Brizel D, Brown E, Eilers JG, et al. NCCN Task Force Report. prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008;6(Suppl 1):S1–21. quiz S2–4
Peterson DE, Bensadoun RJ, Roila F, Group EGW. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi78–84.
Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny AM, Littlewood A, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2011:CD000978.
Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120:1453–61.
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820–31.
Nath CE, Shaw PJ, Trotman J, Zeng L, Duffull SB, Hegarty G, et al. Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Br J Clin Pharmacol. 2010;69:484–97.
Cho YK, Sborov DW, Lamprecht M, Li J, Wang J, Hade EM, et al. Associations of high-dose melphalan pharmacokinetics and outcomes in the setting of a randomized cryotherapy trial. Clin Pharmacol Ther. 2017;102:511–9.
Mori T, Aisa Y, Yamazaki R, Mihara A, Ikeda Y, Okamoto S. Cryotherapy for the prevention of high-dose melphalan-induced oral mucositis. Bone Marrow Transplant. 2006;38:637–8.
Salvador P, Azusano C, Wang L, Howell D. A pilot randomized controlled trial of an oral care intervention to reduce mucositis severity in stem cell transplant patients. J Pain Symptom Manag. 2012;44:64–73.
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
World Health Organization. WHO handbook for reporting results of cancer treatment.. Geneva: World Health Organization; 1979.
Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett WH Jr., Mulagha MT, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer. 1999;85:2103–13.
Grazziutti ML, Dong L, Miceli MH, Krishna SG, Kiwan E, Syed N, et al. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: incidence, risk factors and a severity predictive model. Bone Marrow Transplant. 2006;38:501–6.
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–65.
Rodriguez M, Devlin S, Giralt S, Landau H. Cryotherapy reduces mucositis in multiple myeloma patients receiving high-dose melphalan conditioning prior to autologous stem cell transplantation. Blood. 2012;120:4265.
Batlle M, Morgades M, Vives S, Ferra C, Oriol A, Sancho JM, et al. Usefulness and safety of oral cryotherapy in the prevention of oral mucositis after conditioning regimens with high-dose melphalan for autologous stem cell transplantation for lymphoma and myeloma. Eur J Haematol. 2014;93:487–91.
Marchesi F, Tendas A, Giannarelli D, Viggiani C, Gumenyuk S, Renzi D, et al. Cryotherapy reduces oral mucositis and febrile episodes in myeloma patients treated with high-dose melphalan and autologous stem cell transplant: a prospective, randomized study. Bone Marrow Transplant. 2017;52:154–6.
Acknowledgements
The trial was funded by the Healthcare Committee, Region Västra Götaland (Grant number VGFOUREG-64832). We thank all the patients for their participation in the trial.
Author contributions
J-EJ, who was the chief investigator, and BH, who was the principal investigator, developed the study design. All the authors participated in the planning and performance of the study and in the interpretation of the results. BH, JB, and LH performed the oral assessments. J-EJ identified eligible patients and drafted the manuscript, the final version of which was seen and approved by all contributors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Johansson, JE., Bratel, J., Hardling, M. et al. Cryotherapy as prophylaxis against oral mucositis after high-dose melphalan and autologous stem cell transplantation for myeloma: a randomised, open-label, phase 3, non-inferiority trial. Bone Marrow Transplant 54, 1482–1488 (2019). https://doi.org/10.1038/s41409-019-0468-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0468-6
This article is cited by
-
Oral health, dental treatment, and medication related osteonecrosis of the jaw in multiple myeloma – a longitudinal cohort study
BMC Oral Health (2024)
-
Cryopreventive temperatures prior to chemotherapy
Medical Oncology (2023)
-
Oral cryotherapy for management of chemotherapy‐induced oral mucositis in haematopoietic cell transplantation: a systematic review
BMC Cancer (2022)
-
Clinical trials: design, endpoints and interpretation of outcomes
Bone Marrow Transplantation (2022)
-
Systematic review of oral cryotherapy for the management of oral mucositis in cancer patients and clinical practice guidelines
Supportive Care in Cancer (2020)