Abstract
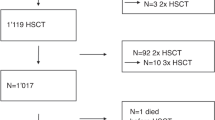
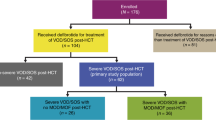
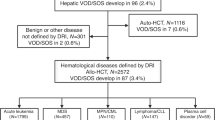
Sirolimus-based graft vs. host disease (GVHD) prophylaxis is associated with higher incidence of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after allogeneic hematopoietic cell transplantation (HCT). However, whether the clinical manifestations and prognosis of VOD/SOS differs when diagnosed in the setting of sirolimus-based GVHD prophylaxis is not well studied. To address this question, we examined presenting features and treatment outcome of VOD/SOS cases identified in a large retrospective cohort of consecutive HCT procedures (n = 818 total, sirolimus (SIR)/tacrolimus (TAC) n = 308, and methotrexate (MTX) or mycophenolate mofetil (MMF)/TAC n = 510). In multivariate analysis, sirolimus-based GVHD prophylaxis (p = 0.006, HR 3.33, 1.94–5.7) increased risk for VOD/SOS. A total of 58 patients were clinically diagnosed with VOD/SOS (SIR/TAC 38/308, 12.3%, vs. MTX or MMF/TAC 20/510, 3.9%). VOD/SOS diagnosed following SIR/TAC prophylaxis demonstrated later time of onset (median 39 vs. 26 days; p = 0.005), less severe hyperbilirubinemia (Bili > 2, 65% vs. 90% p = 0.04), lesser degree of weight gain (weight gain > 5%, 52% vs 80%, p = 0.04), and more frequent complete resolution of hepatic injury (79% vs. 55%, p = 0.05). Presenting features and natural history of VOD/SOS in the context of SIR/TAC GVHD prophylaxis differ and thus warrant particular clinical attention to later hepatic injury in these patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124:1372–7.
Kiel PJ, Vargo CA, Patel GP, Rosenbeck LL, Srivastava S. Possible correlation of sirolimus plasma concentration with sinusoidal obstructive syndrome of the liver in patients undergoing myeloablative allogeneic hematopoietic cell transplantation. Pharmacotherapy. 2012;32:441–5.
Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112:4425–31.
Fukuda D, Sata M, Tanaka K, Nagai R. Potent inhibitory effect of sirolimus on circulating vascular progenitor cells. Circulation. 2005;111:926–31.
Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 2002;16:771–80.
Marks AR. Rapamycin: signaling in vascular smooth muscle. Transplant Proc. 2003;35:231S–3S.
Carmona A, Díaz-Ricart M, Palomo M, Molina P, Pino M, Rovira M, et al. Distinct deleterious effects of cyclosporine and tacrolimus and combined tacrolimus-sirolimus on endothelial cells: protective effect of defibrotide. Biol Blood Marrow Transplant. 2013;19:1439–45.
Khimani F, Kim J, Chen L, Dean E, Rizk V, Betts B, et al. Predictors of overall survival among patients treated with sirolimus/tacrolimus vs methotrexate/tacrolimus for GvHD prevention. Bone Marrow Transplant. 2017;52:1003–9.
Myers KC, Dandoy C, El-Bietar J, Davies SM, Jodele S. Veno-occlusive disease of the liver in the absence of elevation in bilirubin in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:379–81.
Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301–9.
Pai RK, van Besien K, Hart J, Artz AS, O’Donnell PH. Clinicopathologic features of late-onset veno-occlusive disease/sinusoidal obstruction syndrome after high dose intravenous busulfan and hematopoietic cell transplant. Leuk Lymphoma. 2012;53:1552–7.
Shah MS, Jeevangi NKS, Joshi A, Khattry N. Late-onset hepatic veno-occlusive disease post autologous peripheral stem cell transplantation successfully treated with oral defibrotide. J Cancer Res Ther. 2009;5:312–4.
Toh HC, McAfee SL, Sackstein R, Cox BF, Colby C, Spitzer TR. Late onset veno-occlusive disease following high-dose chemotherapy and stem cell transplantation. Bone Marrow Transplant. 1999;24:891–5.
Carreras E, Rosiñol L, Terol MJ, Alegre A, de Arriba F, García-Laraña J, et al. Veno-occlusive disease of the liver after high-dose cytoreductive therapy with busulfan and melphalan for autologous blood stem cell transplantation in multiple myeloma patients. Biol Blood Marrow Transplant. 2007;13:1448–54.
Platzbecker U, von Bonin M, Goekkurt E, Radke J, Binder M, Kiani A, et al. Graft-versus-host disease prophylaxis with everolimus and tacrolimus is associated with a high incidence of sinusoidal obstruction syndrome and microangiopathy: results of the EVTAC trial. Biol Blood Marrow Transplant. 2009;15:101–8.
Rezvani AR, McCune JS, Storer BE, Batchelder A, Kida A, Deeg HJ, et al. Cyclophosphamide followed by intravenous targeted busulfan for allogeneic hematopoietic cell transplantation: pharmacokinetics and clinical outcomes. Biol Blood Marrow Transplant . 2013;19:1033–9.
DeLeve LD, Wang X. Role of oxidative stress and glutathione in busulfan toxicity in cultured murine hepatocytes. Pharmacology. 2000;60:143–54.
DeLeve LD. Chapter 8 - Liver Sinusoidal Endothelial Cells and Liver Injury. Drug-Induced Liver Disease. Third edn. Boston: Academic Press; 2013. pp. 135–46.
Reuben A. Chapter 31 - Hepatotoxicity of Immunosuppressive Drugs. In: Kaplowitz N, DeLeve LD, (eds). Drug-Induced Liver Disease. Third edn. Boston: Academic Press; 2013. pp. 569–91.
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:906–12.
Author contributions
FK, GBMcD and JP contributed in designing the research, data collection, analyzing the data, and writing the manuscript. HS, BB, FL, and HF contributed in research design, critical review, and manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Khimani, F., McDonald, G.B., Shulman, H.M. et al. Hepatic veno-occlusive disease following sirolimus-based immune suppression. Bone Marrow Transplant 54, 85–89 (2019). https://doi.org/10.1038/s41409-018-0233-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0233-2
This article is cited by
-
Feasibility of geriatric assessment before transplant conditioning regimen in older HCT recipients
Bone Marrow Transplantation (2021)