Abstract
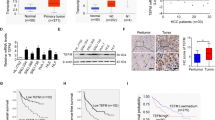
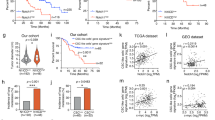
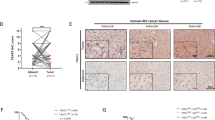
Parkin (PARK2) deficiency is frequently observed in various cancers and potentially promotes tumor progression. Here, we showed that Parkin expression is downregulated in liver cancer tissues, which correlates with poor patient survival. Parkin deficiency in liver cancer cells promotes migration and metastasis as well as changes in EMT and metastasis markers. A negative correlation exists between TMEFF1 and Parkin expression in liver cancer cells and tumor tissues. Parkin deficiency leads to upregulation of TMEFF1 which promotes migration and metastasis. TMEFF1 transcription is activated by Parkin-induced endogenous TGF-β production and subsequent phosphorylation of Smad2/3 and its binding to TMEFF1 promotor. TGF-β inhibitor and TMEFF1 knockdown can reverse shParkin-induced cell migration and changes of EMT markers. Parkin interacts with and promotes the ubiquitin-dependent degradation of HIF-1α/HIF-1β and p53, which accounts for the suppression of TGF-β production. Our data have revealed that Parkin deficiency in cancer leads to the activation of the TGF-β/Smad2/3 pathway, resulting in the expression of TMEFF1 which promotes cell migration, EMT, and metastasis in liver cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–8.
Seirafi M, Kozlov G, Gehring K. Parkin structure and function. FEBS J. 2015;282:2076–88.
Cesari R, Martin ES, Calin GA, Pentimalli F, Bichi R, McAdams H, et al. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc Natl Acad Sci USA. 2003;100:5956–61.
Poulogiannis G, McIntyre RE, Dimitriadi M, Apps JR, Wilson CH, Ichimura K, et al. PARK2 deletions occur frequently in sporadic colorectal cancer and accelerate adenoma development in Apc mutant mice. Proc Natl Acad Sci USA. 2010;107:15145–50.
Picchio MC, Martin ES, Cesari R, Calin GA, Yendamuri S, Kuroki T, et al. Alterations of the tumor suppressor gene Parkin in non-small cell lung cancer. Clin Cancer Res. 2004;10:2720–4.
Denison SR, Wang F, Becker NA, Schüle B, Kock N, Phillips LA, et al. Alterations in the common fragile site gene Parkin in ovarian and other cancers. Oncogene. 2003;22:8370–8.
Yeo CW, Ng FS, Chai C, Tan JM, Koh GR, Chong YK, et al. Parkin pathway activation mitigates glioma cell proliferation and predicts patient survival. Cancer Res. 2012;72:2543–53.
Park KR, Yun JS, Park MH, Jung YY, Yeo IJ, Nam KT, et al. Loss of parkin reduces lung tumor development by blocking p21 degradation. PLoS One. 2019;14:e0217037.
Carroll RG, Hollville E, Martin SJ. Parkin sensitizes toward apoptosis induced by mitochondrial depolarization through promoting degradation of Mcl-1. Cell Rep. 2014;9:1538–53.
Liu J, Zhang C, Zhao Y, Yue X, Wu H, Huang S, et al. Parkin targets HIF-1α for ubiquitination and degradation to inhibit breast tumor progression. Nat Commun. 2017;8:1823.
Yeon M, Bertolini I, Agarwal E, Ghosh JC, Tang HY, Speicher DW, et al. Parkin ubiquitination of Kindlin-2 enables mitochondria-associated metastasis suppression. J Biol Chem. 2023;299:104774.
Li C, Zhang Y, Cheng X, Yuan H, Zhu S, Liu J, et al. PINK1 and PARK2 suppress pancreatic tumorigenesis through control of mitochondrial iron-mediated immunometabolism. Dev Cell. 2018;46:441–55.
Ding D, Ao X, Liu Y, Wang YY, Fa HG, Wang MY, et al. Post-translational modification of Parkin and its research progress in cancer. Cancer Commun. 2019;39:77.
Sun X, Shu Y, Ye G, Wu C, Xu M, Gao R, et al. Histone deacetylase inhibitors inhibit cervical cancer growth through Parkin acetylation-mediated mitophagy. Acta Pharm Sin B. 2022;12:838–52.
Wang F, Denison S, Lai J-P, Philips LA, Montoya D, Kock N, et al. Parkin gene alterations in hepatocellular carcinoma. Genes Chromosomes Cancer. 2004;40:85–96.
Fujiwara M, Marusawa H, Wang HQ, Iwai A, Ikeuchi K, Imai Y, et al. Parkin as a tumor suppressor gene for hepatocellular carcinoma. Oncogene. 2008;27:6002–11.
Gery S, Yin D, Xie D, Black KL, Koeffler HP. TMEFF1 and brain tumors. Oncogene. 2003;22:2723–7.
Nie X, Gao L, Zheng M, Wang C, Wang S, Li X, et al. Overexpression of TMEFF1 in endometrial carcinoma and the mechanism underlying its promotion of malignant behavior in cancer cells. J Cancer. 2021;12:5772–88.
Nie X, Liu C, Guo Q, Zheng MJ, Gao LL, Li X, et al. TMEFF1 overexpression and its mechanism for tumor promotion in ovarian cancer. Cancer Manag Res. 2019;11:839–55.
Su Q, Fan M, Wang J, Ullah A, Ghauri MA, Dai B, et al. Sanguinarine inhibits epithelial–mesenchymal transition via targeting HIF-1α/TGF-β feed-forward loop in hepatocellular carcinoma. Cell Death Dis. 2019;10:939–53.
Wang J, Su Q, Chen K, Wu Q, Ren J, Tang W, et al. Pyrimethamine upregulates BNIP3 to interfere SNARE-mediated autophagosome-lysosomal fusion in hepatocellular carcinoma. J Pharm Anal. 2024;14:211–24.
Chen B, Zhao Y, Lin Z, Liang J, Fan J, Huang Y, et al. Apatinib and gamabufotalin co-loaded lipid/prussian blue nanoparticles for synergistic therapy to gastric cancer with metastasis. J Pharm Anal. 2024; in press. https://doi.org/10.1016/j.jpha.2023.11.011.
Chen Y, Jiang H, Zhan Z, Lu J, Gu T, Yu P, et al. Oridonin restores hepatic lipid homeostasis in an LXRα-ATGL/EPT1 axis-dependent manner. J Pharm Anal. 2023;13:1281–95.
Rauluseviciute I, Riudavets-Puig R, Blanc-Mathieu R, Castro-Mondragon Jaime A, Ferenc K, Kumar V, et al. JASPAR 2024: 20th anniversary of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2023;52:174–82.
Gong Y, Zack TI, Morris LG, Lin K, Hukkelhoven E, Raheja R, et al. Pan-cancer genetic analysis identifies PARK2 as a master regulator of G1/S cyclins. Nat Genet. 2014;46:588–94.
Gong Y, Schumacher SE, Wu WH, Tang F, Beroukhim R, Chan TA. Pan-cancer analysis links PARK2 to BCL-XL-dependent control of apoptosis. Neoplasia. 2017;19:75–83.
Lee MH, Cho Y, Jung BC, Kim SH, Kang YW, Pan CH, et al. Parkin induces G2/M cell cycle arrest in TNF-α-treated HeLa cells. Biochem Biophys Res Commun. 2015;464:63–9.
Veeriah S, Taylor BS, Meng S, Fang F, Yilmaz E, Vivanco I, et al. Somatic mutations of the Parkinson’s disease-associated gene PARK2 in glioblastoma and other human malignancies. Nat Genet. 2010;42:77–82.
Wahabi K, Perwez A, Kamarudheen S, Bhat ZI, Mehta A, Rizvi MMA. Parkin gene mutations are not common, but its epigenetic inactivation is a frequent event and predicts poor survival in advanced breast cancer patients. BMC Cancer. 2019;19:820.
Noelker C, Schwake M, Balzer-Geldsetzer M, Bacher M, Popp J, Schlegel J, et al. Differentially expressed gene profile in the 6-hydroxy-dopamine-induced cell culture model of Parkinson’s disease. Neurosci Lett. 2012;507:10–5.
Eib DW, Holling TM, Zwijsen A, Dewulf N, de Groot E, van den Eijnden-van Raaij AJ, et al. Expression of the follistatin/EGF-containing transmembrane protein M7365 (tomoregulin-1) during mouse development. Mech Dev. 2000;97:167–71.
Moreno-Bueno G, Cubillo E, Sarrió D, Peinado H, Rodríguez-Pinilla SM, Villa S, et al. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543–56.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 81903643 and 82374095), the National Natural Science Foundation of Shaanxi Province (2024JC-YBQN-0891), the Shaanxi Province Science Fund for Distinguished Young Scholars (2023-JC-JQ-59), the Shaanxi Province Science and Technology Development Plan Project (2022ZDLSF05-05). We thank Dr. Wen-juan Tang for reviewing the manuscript.
Author information
Authors and Affiliations
Contributions
QS conceived and designed the experiments and acquired funding. JJW conducted literature search, and worked on the in vitro experiments, animal work, data collection and analysis. JYR, QW, KC, KHT, YZ, AS and XH were involved in animal data collection. SWL, MZ and WFD were involved in review and editing. YMZ is corresponding author, interpreted data and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, Q., Wang, Jj., Ren, Jy. et al. Parkin deficiency promotes liver cancer metastasis by TMEFF1 transcription activation via TGF-β/Smad2/3 pathway. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01254-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01254-3