Abstract
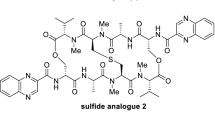
The development of targeted chemotherapeutic agents against colorectal cancer (CRC), one of the most common cancers with a high mortality rate, is in a constant need. Nannocystins are a family of myxobacterial secondary metabolites featuring a 21-membered depsipeptide ring. The in vitro anti-CRC activity of natural and synthetic nannocystins was well documented, but little is known about their in vivo efficacy and if positive, the underlying mechanism of action. In this study we synthesized a nitroaromatic nannocystin through improved preparation of a key fragment, and characterized its in vitro activity and in vivo efficacy against CRC. We first described the total synthesis of compounds 2–4 featuring Heck macrocyclization to forge their 21-membered macrocycle. In a panel of 7 cancer cell lines from different tissues, compound 4 inhibited the cell viability with IC values of 1–6 nM. In particular, compound 4 (1, 2, 4 nM) inhibited the proliferation of CRC cell lines (HCT8, HCT116 and LoVo) in both concentration and time dependent manners. Furthermore, compound 4 concentration-dependently inhibited the colony formation and migration of CRC cell lines. Moreover, compound 4 induced cell cycle arrest at sub-G1 phase, apoptosis and cellular senescence in CRC cell lines. In three patient-derived CRC organoids, compound 4 inhibited the PDO with IC values of 3.68, 28.93 and 11.81 nM, respectively. In a patient-derived xenograft mouse model, injection of compound 4 (4, 8 mg/kg, i.p.) every other day for 12 times dose-dependently inhibited the tumor growth without significant change in body weight. We conducted RNA-sequencing, molecular docking and cellular thermal shift assay to elucidate the anti-CRC mechanisms of compound 4, and revealed that it exerted its anti-CRC effect at least in part by targeting AKT1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80.
Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79–92.
Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Sig Transduct Target Ther. 2020;5:22.
Cassidy S, Syed BA. Colorectal cancer drugs market. Nat Rev Drug Discov. 2017;16:525–6.
Deshmukh R, Prajapati M, Harwansh RK. A review on emerging targeted therapies for the management of metastatic colorectal cancers. Med Oncol. 2023;40:159.
Hoffmann H, Kogler H, Heyse W, Matter H, Caspers M, Schummer D, et al. Discovery, structure elucidation, and biological characterization of nannocystin A, a macrocyclic myxobacterial metabolite with potent antiproliferative properties. Angew Chem Int Ed. 2015;54:10145–8.
Krastel P, Roggo S, Schirle M, Ross NT, Perruccio F, Aspesi P Jr, et al. Nannocystin A: an elongation factor 1 inhibitor from myxobacteria with differential anti-cancer properties. Angew Chem Int Ed. 2015;54:10149–54.
Yang Z, Xu X, Yang CH, Tian Y, Chen X, Lian L, et al. Total synthesis of nannocystin A. Org Lett. 2016;18:5768–70.
Tian Y, Xu X, Ding Y, Hao X, Bai Y, Tang Y, et al. Synthesis and biological evaluation of nannocystin analogues toward understanding the binding role of the (2R,3S)-epoxide in nannocystin A. Eur J Med Chem. 2018;150:626–32.
Tian Y, Ding Y, Xu X, Bai Y, Tang Y, Hao X, et al. Total synthesis and biological evaluation of nannocystin analogues modified at the polyketide phenyl moiety. Tetrahedron Lett. 2018;59:3206–9.
Tian Y, Wang J, Liu W, Yuan X, Tang Y, Li J, et al. Stereodivergent total synthesis of Br-nannocystins underpinning the polyketide (10R,11S) configuration as a key determinant of potency. J Mol Struct. 2019;1181:568–78.
Zhang H, Tian Y, Yuan X, Xie F, Yu S, Cai J, et al. Site-directed late-stage diversification of macrocyclic nannocystins facilitating anticancer SAR and mode of action studies. RSC Med Chem. 2023;14:299–312.
Meng Z, Souillart L, Monks B, Huwyler N, Herrmann J, Mueller R, et al. A “Motif-Oriented” total synthesis of nannocystin Ax. Preparation and biological assessment of analogues. J Org Chem. 2018;83:6977–94.
Miyakita D, Kawanishi K, Katsuyama A, Yamamoto K, Yakushiji F, Ichikawa S. Solid-phase synthesis of nannocystin Ax and its analogues. J Org Chem. 2023;88:11367–71.
Nomula R, Pratapure MS, Kontham R. Studies directed toward the total synthesis of nannocystin A. ChemistrySelect. 2022;7:e202203893.
Liu Q, Yang X, Ji J, Zhang SL, He Y. Novel nannocystin A analogues as anticancer therapeutics: synthesis, biological evaluations and structure-activity relationship studies. Eur J Med Chem. 2019;170:99–111.
Hou Y, Liu R, Xia M, Sun C, Zhong B, Yu J, et al. Nannocystin ax, an eEF1A inhibitor, induces G1 cell cycle arrest and caspase-independent apoptosis through cyclin D1 downregulation in colon cancer in vivo. Pharmacol Res. 2021;173:105870.
Sun C, Liu R, Xia M, Hou Y, Wang X, Lu JJ, et al. Nannocystin Ax, a natural elongation factor 1α inhibitor from Nannocystis sp., suppresses epithelial-mesenchymal transition, adhesion and migration in lung cancer cells. Toxicol Appl Pharmacol. 2021;420:115535.
Paul D, Das S, Saha S, Sharma H, Goswami RK. Intramolecular heck reaction in total synthesis of natural products: an update. Eur J Org Chem. 2021;2021:2057–76.
Zhang W. Heck macrocyclization in natural product total synthesis. Nat Prod Rep. 2021;38:1109–35.
Cai J, Sun B, Yu S, Zhang H, Zhang W. Heck macrocyclization in forging non-natural large rings including macrocyclic drugs. Int J Mol Sci. 2023;24:8252.
Glaus F, Altmann KH. Total synthesis of the tiacumicin B (Lipiarmycin A3/Fidaxomicin) aglycone. Angew Chem Int Ed. 2015;54:1937–40.
Shirokawa S-I, Kamiyama M, Nakamura T, Okada M, Nakazaki A, Hosokawa S, et al. Remote asymmetric induction with vinylketene silyl N,O-acetal. J Am Chem Soc. 2004;126:13604–5.
Kalesse M, Cordes M, Symkenberg G, Lu HH. The vinylogous Mukaiyama aldol reaction (VMAR) in natural product synthesis. Nat Prod Rep. 2014;31:563–94.
Hosokawa S. Remote asymmetric induction reactions using a E,E-vinylketene silyl N,O-acetal and the wide range stereocontrol strategy for the synthesis of polypropionates. Acc Chem Res. 2018;51:1301–14.
Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer. Mol Med Rep. 2015;11:1566–72.
Wickenden JA, Watson CJ. Key signalling nodes in mammary gland development and cancer. Signalling downstream of PI3 kinase in mammary epithelium: a play in 3 Akts. Breast Cancer Res. 2010;12:202.
Thompson BJ. YAP/TAZ: drivers of tumor growth, metastasis, and resistance to therapy. Bioessays. 2020;42:1900162.
Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573:439–44.
Paulin D, Lilienbaum A, Kardjian S, Agbulut O, Li Z. Vimentin: regulation and pathogenesis. Biochimie. 2022;197:96–112.
Cargnello M, Tcherkezian J, Roux PP. The expanding role of mTOR in cancer cell growth and proliferation. Mutagenesis. 2015;30:169–76.
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997–2007.
Pinzi L, Rastelli G. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci. 2019;20:4331.
Molina DM, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science. 2013;341:84–7.
Caballero IM, Lundgren S. A shift in thinking: cellular thermal shift assay-enabled drug discovery. ACS Med Chem Lett. 2023;14:369–75.
Lin R, Elf S, Shan C, Kang H-B, Ji Q, Zhou L, et al. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1–AMPK signalling. Nat Cell Biol. 2015;17:1484–96.
Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–67.
Degan SE, Gelman IH. Emerging roles for AKT isoform preference in cancer progression pathways. Mol Cancer Res. 2021;19:1251–7.
Doak BC, Over B, Giordanetto F, Kihlberg J. Oral druggable space beyond the rule of 5: insights from drugs and clinical candidates. Chem Biol. 2014;21:1115–42.
Doak BC, Zheng J, Dobritzsch D, Kihlberg J. How beyond rule of 5 drugs and clinical candidates bind to their targets. J Med Chem. 2016;59:2312–27.
Matsson P, Doak BC, Over B, Kihlberg J. Cell permeability beyond the rule of 5. Adv Drug Deliv Rev. 2016;101:42–61.
Poongavanam V, Doak BC, Kihlberg J. Opportunities and guidelines for discovery of orally absorbed drugs in beyond rule of 5 space. Curr Opin Chem Biol. 2018;44:23–9.
Tyagi M, Begnini F, Poongavanam V, Doak BC, Kihlberg J. Drug syntheses beyond the rule of 5. Chem Eur J. 2020;26:49–88.
Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery—an underexploited structural class. Nat Rev Drug Discov. 2008;7:608–24.
Marsault E, Peterson ML. Macrocycles are great cycles: applications, opportunities, and challenges of synthetic macrocycles in drug discovery. J Med Chem. 2011;54:1961–2004.
Giordanetto F, Kihlberg J. Macrocyclic drugs and clinical candidates: what can medicinal chemists learn from their properties? J Med Chem. 2014;57:278–95.
Amrhein JA, Knapp S, Hanke T. Synthetic opportunities and challenges for macrocyclic kinase inhibitors. J Med Chem. 2021;64:7991–8009.
Garcia Jimenez D, Poongavanam V, Kihlberg J. Macrocycles in drug discovery─learning from the past for the future. J Med Chem. 2023;66:5377–96.
Zhang W. From target-oriented to motif-oriented: a case study on nannocystin total synthesis. Molecules. 2020;25:5327.
Liao L, Zhou J, Xu Z, Ye T. Concise total synthesis of nannocystin A. Angew Chem Int Ed. 2016;55:13263–6.
Zhang YH, Liu R, Liu B. Total synthesis of nannocystin Ax. Chem Commun. 2017;53:5549–52.
Baker R, Castro JL. Total synthesis of (+)-macbecin I. J Chem Soc Perkin Trans 1. 1990:47–65.
Menche D, Hassfeld J, Li J, Rudolph S. Total synthesis of archazolid A. J Am Chem Soc. 2007;129:6100–1.
Mailhol D, Willwacher J, Kausch-Busies N, Rubitski EE, Sobol Z, Schuler M, et al. Synthesis, molecular editing, and biological assessment of the potent cytotoxin leiodermatolide. J Am Chem Soc. 2014;136:15719–29.
Li X, Zeng X. Sequential trans-halogenation and Heck reaction for efficient access to dioxo-tetra-substituted 2,4 E,E-dienes: synthesis of segment C1-C6 of apoptolidin. Tetrahedron Lett. 2006;47:6839–42.
Schmitt CA. Senescence, apoptosis and therapy—cutting the lifelines of cancer. Nat Rev Cancer. 2003;3:286–95.
Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18:407–18.
Weeber F, Ooft SN, Dijkstra KK, Voest EE. Tumor organoids as a pre-clinical cancer model for drug discovery. Cell Chem Biol. 2017;24:1092–100.
Wensink GE, Elias SG, Mullenders J, Koopman M, Boj SF, Kranenburg OW, et al. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precision Onc. 2021;5:30.
Kopetz S, Lemos R, Powis G. The promise of patient-derived xenografts: the best laid plans of mice and men. Clin Cancer Res. 2012;18:5160–2.
Julien S, Merino-Trigo A, Lacroix L, Pocard M, Goéré D, Mariani P, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;18:5314–28.
Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405.
Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372–83.
Mundi PS, Sachdev J, McCourt C, Kalinsky K. AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol. 2016;82:943–56.
Hua H, Zhang H, Chen J, Wang J, Liu J, Jiang Y. Targeting Akt in cancer for precision therapy. J Hematol Oncol. 2021;14:128.
Huang J, Chen L, Wu J, Ai D, Zhang JQ, Chen TG, et al. Targeting the PI3K/AKT/mTOR signaling pathway in the treatment of human diseases: current status, trends, and solutions. J Med Chem. 2022;65:16033–61.
Palmer AC, Kishony R. Opposing effects of target overexpression reveal drug mechanisms. Nat Commun. 2014;5:4296.
Roy R, Winteringham LN, Lassmann T, Forrest AR. Expression levels of therapeutic targets as indicators of sensitivity to targeted therapeutics. Mol Cancer Ther. 2019;18:2480–9.
Ferguson FM, Gray NS. Kinase inhibitors: the road ahead. Nat Rev Drug Discov. 2018;17:353–77.
Lu X, Smaill JB, Ding K. Medicinal chemistry strategies for the development of kinase inhibitors targeting point mutations. J Med Chem. 2020;63:10726–41.
Lu X, Smaill JB, Patterson AV, Ding K. Discovery of cysteine-targeting covalent protein kinase inhibitors. J Med Chem. 2022;65:58–83.
Amiri A, Noei F, Jeganathan S, Kulkarni G, Pinke DE, Lee JM. eEF1A2 activates Akt and stimulates Akt-dependent actin remodeling, invasion and migration. Oncogene. 2007;26:3027–40.
Xu C, Hu DM, Zhu Q. eEF1A2 promotes cell migration, invasion and metastasis in pancreatic cancer by upregulating MMP-9 expression through Akt activation. Clin Exp Metastasis. 2013;30:933–44.
Pellegrino R, Calvisi DF, Neumann O, Kolluru V, Wesely J, Chen X, et al. EEF1A2 inactivates p53 by way of PI3K/AKT/mTOR-dependent stabilization of MDM4 in hepatocellular carcinoma. Hepatology. 2014;59:1886–99.
Qiu FN, Huang Y, Chen DY, Li F, Wu YA, Wu WB, et al. Eukaryotic elongation factor-1α2 knockdown inhibits hepatocarcinogenesis by suppressing PI3K/Akt/NF-κB signaling. World J Gastroenterol. 2016;22:4226–37.
Hassan MK, Kumar D, Patel SA, Pattanaik N, Mohapatra N, Dixit M. Expression pattern of EEF1A2 in brain tumors: histological analysis and functional role as a promoter of EMT. Life Sci. 2020;246:117399.
Yang J, Tang J, Li J, Cen Y, Chen J, Dai G. Effect of activation of the Akt/mTOR signaling pathway by EEF1A2 on the biological behavior of osteosarcoma. Ann Transl Med. 2021;9:158.
Zhang H, Cai J, Yu S, Sun B, Zhang W. Anticancer small-molecule agents targeting eukaryotic elongation factor 1A: state of the art. Int J Mol Sci. 2023;24:5184.
Potts MB, McMillan EA, Rosales TI, Kim HS, Ou Y-H, Toombs JE, et al. Mode of action and pharmacogenomic biomarkers for exceptional responders to didemnin B. Nat Chem Biol. 2015;11:401–8.
Liu C, Wang L, Sun Y, Zhao X, Chen T, Su X, et al. Probe synthesis reveals eukaryotic translation elongation factor 1 alpha 1 as the anti-pancreatic cancer target of BE-43547A2. Angew Chem Int Ed. 2022;61:e202206953.
Villadsen NL, Jacobsen KM, Keiding UB, Weibel ET, Christiansen B, Vosegaard T, et al. Synthesis of ent-BE-43547A1 reveals a potent hypoxia-selective anticancer agent and uncovers the biosynthetic origin of the APD-CLD natural products. Nat Chem. 2017;9:264–72.
Jacobsen KM, Villadsen NL, Toerring T, Nielsen CB, Salomon T, Nielsen MM, et al. APD-containing cyclolipodepsipeptides target mitochondrial function in hypoxic cancer cells. Cell Chem Biol. 2018;25:1337–49.
Frankowski KJ, Wang C, Patnaik S, Schoenen FJ, Southall N, Li D, et al. Metarrestin, a perinucleolar compartment inhibitor, effectively suppresses metastasis. Sci Transl Med. 2018;10:eaap8307.
Nepali K, Lee HY, Liou JP. Nitro-group-containing drugs. J Med Chem. 2019;62:2851–93.
Acknowledgements
This work was generously supported by the National Natural Science Foundation of China (NNSFC) (No. 21772101) to WCZ, the National Natural Science Foundation of China (NNSFC) (Nos. 81973356 and 82373906) to CLS, and the Fundamental Research Funds for the Central Universities in Nankai University (Nos. 63231108, ZB22010404, and 63221331) to CLS.
Author information
Authors and Affiliations
Contributions
WCZ and CLS designed the study. HZ, FX, XYY, YFT, XTD, MMS, SQY, JYC and BS performed the experiments. WCZ and CLS wrote the manuscript. HZ, FX, WCZ and CLS revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A patent on behalf of Nankai University has been filed.
Ethical approval
This study was carried out in accordance with the recommendations of Requirements of the Ethical Review System of Biomedical Research Involving Human by National Health and Family Planning Commission of China, Nankai University Laboratory Animal Welfare Ethics Review Committee (Approval No. 2023-SYDWLL-000066), Biomedical Ethics Committee of Nankai University (Ethics Document No. NKUIRB2023024), and Tianjin Central Hospital of Gynecology Obstetrics with written informed consent from all subjects. All subjects have been given a written informed consent in accordance with the Declaration of Helsinki.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Xie, F., Yuan, Xy. et al. Discovery of a nitroaromatic nannocystin with potent in vivo anticancer activity against colorectal cancer by targeting AKT1. Acta Pharmacol Sin 45, 1044–1059 (2024). https://doi.org/10.1038/s41401-024-01231-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-024-01231-w