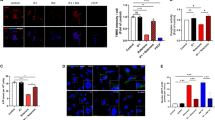
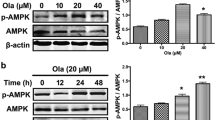
Abstract
Dopaminergic neurons in the substantia nigra (SN) expressing SUR1/Kir6.2 type ATP-sensitive potassium channels (K-ATP) are more vulnerable to rotenone or metabolic stress, which may be an important reason for the selective degeneration of neurons in Parkinson’s disease (PD). Baicalein has shown neuroprotective effects in PD animal models. In this study, we investigated the effect of baicalein on K-ATP channels and the underlying mechanisms in rotenone-induced apoptosis of SH-SY5Y cells. K-ATP currents were recorded from SH-SY5Y cells using whole-cell voltage-clamp recording. Drugs dissolved in the external solution at the final concentration were directly pipetted onto the cells. We showed that rotenone and baicalein opened K-ATP channels and increased the current amplitudes with EC50 values of 0.438 μM and 6.159 μM, respectively. K-ATP channel blockers glibenclamide (50 μM) or 5-hydroxydecanoate (5-HD, 250 μM) attenuated the protective effects of baicalein in reducing reactive oxygen species (ROS) content and increasing mitochondrial membrane potential and ATP levels in rotenone-injured SH-SY5Y cells, suggesting that baicalein protected against the apoptosis of SH-SY5Y cells by regulating the effect of rotenone on opening K-ATP channels. Administration of baicalein (150, 300 mg·kg−1·d−1, i.g.) significantly inhibited rotenone-induced overexpression of SUR1 in SN and striatum of rats. We conducted surface plasmon resonance assay and molecular docking, and found that baicalein had a higher affinity with SUR1 protein (KD = 10.39 μM) than glibenclamide (KD = 24.32 μM), thus reducing the sensitivity of K-ATP channels to rotenone. Knockdown of SUR1 subunit reduced rotenone-induced apoptosis and damage of SH-SY5Y cells, confirming that SUR1 was an important target for slowing dopaminergic neuronal degeneration in PD. Taken together, we demonstrate for the first time that baicalein attenuates rotenone-induced SH-SY5Y cell apoptosis through binding to SUR1 and activating K-ATP channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323:548–60.
Dorsey ER, Elbaz A, Nichols E, Abd-Allah F, Abdelalim A, C.Adsuar J, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–53.
Vázquez-Vélez GE, Zoghbi HY. Parkinson’s disease genetics and pathophysiology. Annu Rev Neurosci. 2021;44:87–108.
Daniel NH, Aravind A, Thakur P. Are ion channels potential therapeutic targets for Parkinson’s disease? Neurotoxicology. 2021;87:243–57.
Liu M, Liu C, Xiao X, Han SS, Bi MX, Jiao Q, et al. Role of upregulation of the K(ATP) channel subunit SUR1 in dopaminergic neuron degeneration in Parkinson’s disease. Aging Cell. 2022;21:e13618.
Zhao S, Wang M, Ma Z. Therapeutic potential of ATP-sensitive potassium channels in Parkinson’s disease. Brain Res Bull. 2021;169:1–7.
Minami K, Miki T, Kadowaki T, Seino S. Roles of ATP-sensitive K+ channels as metabolic sensors: studies of Kir6.x null mice. Diabetes. 2004;53:S176–80.
Yee AG, Freestone PS, Bai JZ, Lipski J. Paradoxical lower sensitivity of Locus Coeruleus than Substantia Nigra pars compacta neurons to acute actions of rotenone. Exp Neurol. 2017;287:34–43.
Liss B, Bruns R, Roeper J. Alternative sulfonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurons. EMBO J. 1999;18:833–46.
Schiemann J, Schlaudraff F, Klose V, Bingmer M, Seino S, Magill PJ, et al. K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat Neurosci. 2012;15:1272–80.
Han SS, Jiao Q, Bi MX, Du XX, Jiang H. The expression of K(ATP) channel subunits in alpha-synuclein-transfected MES23.5 cells. Ann Transl Med. 2018;6:170.
Pang H, Xue W, Shi A, Li M, Li Y, Cao G, et al. Multiple-ascending-dose pharmacokinetics and safety evaluation of baicalein chewable tablets in healthy chinese volunteers. Clin Drug Investig. 2016;36:713–24.
Tarragó T, Kichik N, Claasen B, Prades R, Teixidó M, Giralt E. Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorg Med Chem. 2008;16:7516–24.
Tsai PL, Tsai TH. Pharmacokinetics of baicalin in rats and its interactions with cyclosporin A, quinidine and SKF-525A: a microdialysis study. Planta Med. 2004;70:1069–74.
Li Y, Zhao J, Hölscher C. Therapeutic potential of baicalein in Alzheimer’s disease and Parkinson’s disease. CNS Drugs. 2017;31:639–52.
Rui W, Li S, Xiao H, Xiao M, Shi J. Baicalein attenuates neuroinflammation by inhibiting NLRP3/caspase-1/GSDMD pathway in MPTP induced mice model of Parkinson’s disease. Int J Neuropsychopharmacol. 2020;23:762–73.
Wang Y, Wei N, Li X. Preclinical evidence and possible mechanisms of baicalein for rats and mice with Parkinson’s disease: a systematic review and meta-analysis. Front Aging Neurosci. 2020;12:277.
Sonawane SK, Uversky VN, Chinnathambi S. Baicalein inhibits heparin-induced Tau aggregation by initializing non-toxic Tau oligomer formation. Cell Commun Signal. 2021;19:16.
Zhao X, Kong D, Zhou Q, Wei G, Song J, Liang Y, et al. Baicalein alleviates depression-like behavior in rotenone- induced Parkinson’s disease model in mice through activating the BDNF/TrkB/CREB pathway. Biomed Pharmacother. 2021;140:111556.
Chen M, Peng L, Gong P, Zheng X, Sun T, Zhang X, et al. Baicalein mediates mitochondrial autophagy via miR-30b and the NIX/BNIP3 signaling pathway in Parkinson’s disease. Biochem Res Int. 2021;2021:2319412.
Song Q, Peng S, Zhu X. Baicalein protects against MPP+/MPTP-induced neurotoxicity by ameliorating oxidative stress in SH-SY5Y cells and mouse model of Parkinson’s disease. Neurotoxicology. 2021;87:188–94.
Liu RZ, Zhang S, Zhang W, Zhao XY, Du GH. Baicalein attenuates brain iron accumulation through protecting aconitase 1 from oxidative stress in rotenone-induced Parkinson’s disease in Rats. Antioxidants (Basel). 2022;12:12.
Wang IC, Lin JH, Lee WS, Liu CH, Lin TY, Yang KT. Baicalein and luteolin inhibit ischemia/reperfusion-induced ferroptosis in rat cardiomyocytes. Int J Cardiol. 2023;375:74–86.
Du X, Xu H, Shi L, Jiang Z, Song N, Jiang H, et al. Activation of ATP-sensitive potassium channels enhances DMT1-mediated iron uptake in SK-N-SH cells in vitro. Sci Rep. 2016;6:33674.
Kampa RP, Sęk A, Bednarczyk P, Szewczyk A, Calderone V, Testai L. Flavonoids as new regulators of mitochondrial potassium channels: contribution to cardioprotection. J Pharm Pharmacol. 2023;75:466–81.
Zhang X, Du L, Zhang W, Yang Y, Zhou Q, Du G. Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci Rep. 2017;7:9968.
Liu J, Zheng X, Li W, Ren L, Li S, Yang Y, et al. Anti-tumor effects of Skp2 inhibitor AAA-237 on NSCLC by arresting cell cycle at G0/G1phase and inducing senescence. Pharmacol Res. 2022;181:106259.
Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29:323–33.
Martin GM, Sung MW, Yang Z, Innes LM, Kandasamy B, David LL, et al. Mechanism of pharmacochaperoning in a mammalian KATP channel revealed by cryo-EM. Elife. 2019;8:e46417.
Bai Q, He J, Qiu J, Wang Y, Wang S, Xiu Y, et al. Rotenone induces KATP channel opening in PC12 cells in association with the expression of tyrosine hydroxylase. Oncol Rep. 2012;28:1376–84.
Zhang Z, Shi L, Du X, Jiao Q, Jiang H. Acute action of rotenone on excitability of catecholaminergic neurons in rostral ventrolateral medulla. Brain Res Bull. 2017;134:151–61.
Wu C, Yang K, Liu Q, Wakui M, Jin GZ, Zhen X, et al. Tetrahydroberberine blocks ATP-sensitive potassium channels in dopamine neurons acutely-dissociated from rat substantia nigra pars compacta. Neuropharmacology. 2010;59:567–72.
Xie J, Duan L, Qian X, Huang X, Ding J, Hu G. K(ATP) channel openers protect mesencephalic neurons against MPP+-induced cytotoxicity via inhibition of ROS production. J Neurosci Res. 2010;88:428–37.
Liu X, Wu JY, Zhou F, Sun XL, Yao HH, Yang Y, et al. The regulation of rotenone-induced inflammatory factor production by ATP-sensitive potassium channel expressed in BV-2 cells. Neurosci Lett. 2006;394:131–5.
Fan Y, Kong H, Ye X, Ding J, Hu G. ATP-sensitive potassium channels: uncovering novel targets for treating depression. Brain Struct Funct. 2016;221:3111–22.
Yee AG, Lee SM, Hunter MR, Glass M, Freestone PS, Lipski J. Effects of the Parkinsonian toxin MPP+ on electrophysiological properties of nigral dopaminergic neurons. Neurotoxicology. 2014;45:1–11.
Lawson K. Is there a role for potassium channel openers in neuronal ion channel disorders? Expert Opin Investig Drugs. 2000;9:2269–80.
Lv J, Xiao X, Bi M, Tang T, Kong D, Diao M, et al. ATP-sensitive potassium channels: a double-edged sword in neurodegenerative diseases. Ageing Res Rev. 2022;80:101676.
Singh A, Tripathi P, Yadawa AK, Singh S. Promising polyphenols in Parkinson’s disease therapeutics. Neurochem Res. 2020;45:1731–45.
Alquisiras-Burgos I, Franco-Pérez J, Rubio-Osornio M, Aguilera P. The short form of the SUR1 and its functional implications in the damaged brain. Neural Regen Res. 2022;17:488–96.
Zhang Q, Li C, Zhang T, Ge Y, Han X, Sun S. Deletion of Kir6.2/SUR1 potassium channels rescues diminishing of DA neurons via decreasing iron accumulation in PD. Mol Cell Neurosci. 2018;92:164–76.
Chen MM, Hu ZL, Ding JH, Du RH, Hu G. Astrocytic Kir6.1 deletion aggravates neurodegeneration in the lipopolysaccharide-induced mouse model of Parkinson’s disease via astrocyte-neuron cross talk through complement C3-C3R signaling. Brain Behav Immun. 2021;95:310–20.
Hu ZL, Sun T, Lu M, Ding JH, Du RH, Hu G. Kir6.1/K-ATP channel on astrocytes protects against dopaminergic neurodegeneration in the MPTP mouse model of Parkinson’s disease via promoting mitophagy. Brain Behav Immun. 2019;81:509–22.
Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci. 2005;8:1742–51.
Acknowledgements
This work was supported by the Beijing Natural Science Foundation (7232258) and the CAMS Innovation Fund for Medical Sciences (Nos. 2021-I2M-1-005, 2022-I2M-JB-010).
Author information
Authors and Affiliations
Contributions
DWK performed most of the experiments, analyzed the data and wrote the draft. LDD and RZL assisted in animal experiments. TYY and SBW carried out the Western blotting. YHW and YL provided baicalein (98% purity, crystal β form). LHF and GHD designed the project and revised the draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, Dw., Du, Ld., Liu, Rz. et al. Baicalein attenuates rotenone-induced SH-SY5Y cell apoptosis through binding to SUR1 and activating ATP-sensitive potassium channels. Acta Pharmacol Sin 45, 480–489 (2024). https://doi.org/10.1038/s41401-023-01187-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01187-3