Abstract
High dose intake of docosahexaenoic acid showed beneficial effects on atopic dermatitis in patients and was found to increase regulatory T cells in mice, but its molecular target has not been identified. Free fatty acid receptor 4 (FFA4, also known as GPR120) is a receptor sensing polyunsaturated long-chain fatty acids including docosahexaenoic acid. In the present study, we examined whether FFA4 acted as a therapeutic target of docosahexaenoic acid for treating atopic dermatitis. Experimental atopic dermatitis was induced in mice by 2,4-dinitrochlorobenzene (DNCB) sensitization on day 0, followed by repeated DNCB challenges from D7 to D48. The mice were treated with a selective agonist compound A (30 mg· kg−1· d−1, ip) from D19 to D48, and sacrificed on D49. We found that DNCB-induced atopic dermatitis-like skin lesions, i.e. hypertrophy and mast cell infiltration in skin tissues, as well as markedly elevated serum IgE levels. Administration of compound A significantly suppressed the atopic responses in ears and lymph nodes, such as hypertrophy and mast cell infiltration in the ears, enlarged sizes of lymph nodes, and elevated serum IgE and levels of cytokines IL-4, IL-13, IL-17, and IFN-γ in ear tissue. The therapeutic effects of compound A were abolished by FFA4 knockout. Similarly, increased CD4+Foxp3+ regulatory T-cell population in lymph nodes was observed in wide-type mice treated with compound A, but not seen in FFA4-deficient mice. In conclusion, we demonstrate that activation of FFA4 ameliorates atopic dermatitis by increasing CD4+Foxp3+ regulatory T cells, suggesting FFA4 as a therapeutic target for atopic dermatitis.
Similar content being viewed by others
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin disorder characterized by intense pruritus and eczematous patches or plaques with a complex etiology [1]. AD affects up to 20% of children worldwide and can significantly compromise quality of life due to emotional distress, sleep disruption, and social awkwardness [2]. The pathogenesis of AD is associated with skin barrier dysfunction and allergic inflammation. Antigen-presenting cells, such as Langerhans cells, in the skin are activated when they make contacts with allergens. Dendritic cells promote the differentiation of naive T cells into Th2 cells in lymph nodes [3]. Increased levels of Th2 cytokines (IL-4 and IL-13), IFN-γ and TNF-α not only promote the apoptosis of keratinocytes but also increase thymic stromal lymphopoietin expression in epithelial cells, leading to additional damage to the skin barrier [4].
Omega-3 polyunsaturated fatty acids, including docosahexaenoic acid (DHA; C22:6), eicosapentaenoic acid (EPA; C20:5), and α-linolenic acid (C18:3), play an important role in controlling inflammation and tissue homeostasis [5, 6]. Omega-3 polyunsaturated fatty acids cannot be synthesized by humans, so it is essential to obtain them through the diet. As a dietary intervention, high amounts of DHA supplementation were shown to have a beneficial impact on the outcome of AD in a pilot trial in humans [7]. Later, DHA supplementation was confirmed to suppress the development of AD in mice and found to induce M2 macrophage polarization and CD4+Foxp3+ regulatory T-cell generation to increase the suppression of atopic inflammation [8]. However, the molecular target of DHA was not identified.
Free fatty acid receptor 4 (FFA4, also known as GPR120) is a specific receptor for omega-3 polyunsaturated fatty acids [9, 10]. FFA4 has been shown to be expressed in the large intestine, adipocytes, and lungs and has also been reported to be present in many tissues that play an important role in inflammation and immune responses [5, 11]. The expression of FFA4 has been reported in immune cells, including monocytes, macrophages, dendritic cells, and eosinophils [12,13,14]. FFA4-mediated M2 macrophage induction in vivo was shown to be a beneficial mechanism involved in obesity-related metabolic inflammation [10, 15]. In a naphthalene-induced acute airway injury model, recovery was accelerated by FFA4 activation in lung epithelial club cells, and club cell proliferation and migration were also stimulated by FFA4 [16]. In addition, FFA4 activation inhibited hepatic steatosis of hepatocytes in a nonalcoholic fatty liver disease model induced by a high-fat diet [17]. However, the role of FFA4 in AD has not been studied.
Compound A (3-[2-chloro-5-(trifluoromethoxy)phenyl]-3-azaspiro[5.5]undecane-9-acetic acid) is a selective, high-affinity, orally available, and spirocyclic FFA4 agonist [15, 18]. Compound A exerts potent anti-inflammatory effects on macrophages in vitro and in obese mice in vivo [15]. Treatment with compound A increased the number of anti-inflammatory M2-phenotype macrophages and Foxp3+ regulatory T cells in the adipose tissue of high-fat diet-induced obese mice [15]. Thus, if compound A inhibits allergic inflammation in AD, it may have additional therapeutic effects that increase the levels of regulatory T cells that play a critical role in maintaining tolerance to self-antigens and regulating the immune response [19]. Hence, the aim of this study was to examine whether FFA4 has anti-inflammatory activity in AD and promotes the generation of regulatory T cells. In the present study, therefore, we utilized a 2,4-dinitrochlorobenzene (DNCB)-induced AD model in combination with compound A, which is a selective agonist of FFA4, and FFA4-deficient mice [15,16,17, 20, 21].
Materials and methods
Materials
Compound A was purchased from Cayman Chemical (Ann Arbor, MI, USA). Acetone was obtained from Junsei Chemical (Tokyo, Japan). 2,4-DNCB and olive oil were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animals
FFA4 knockout mice (TF0224) were purchased from Lexicon Pharmaceuticals (Woodlands, TX, USA); these mice were backcrossed to BALB/c mice for eight generations [16]. All animals were housed in a laboratory animal facility at Pusan National University and provided food and water ad libitum. Two mice were housed per cage in standard plastic cages with sawdust as bedding and maintained under controlled conditions with a temperature of 22–24 °C, humidity of 60% ± 5%, and alternating light/dark cycles (lights were on between 7:00 and 19:00), and the mice were provided with standard laboratory chow and water. The animal protocol used was reviewed by the Pusan National University–Institutional Animal Care Committee with respect to ethicality of the procedures used and care provided (Approval Number PNU-2019-2218).
Induction of AD in BALB/c mice and compound A administration
According to a simple randomization procedure, 8-week-old male WT BALB/c and KO mice were divided into six groups (n = 5): a vehicle (acetone:olive oil, 3:1)-treated control FFA4 WT group, a DNCB-treated FFA4 WT mouse group, a compound A/DNCB-treated FFA4 WT group, a vehicle-treated control FFA4 KO group, a DNCB-treated FFA4 KO mouse group, and a compound A/DNCB-treated FFA4 KO group. To induce experimental AD, the ventral skin was shaved, and 300 μL of 1% DNCB in acetone/olive oil (3:1) was applied to the ventral skin on day 0 (Fig. 1a). On day 7, mice were challenged again by application of 200 μL of 0.3% DNCB to the ears every other day for up to 48 days. From day 19 until the completion of the experiment, the compound A/DNCB-treated FFA4 WT group was administered an intraperitoneal injection of compound A (30 mg/kg body weight) 30 min before the challenge. The mice were sacrificed on day 49 [8].
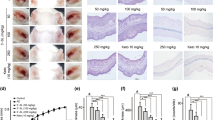
a Experimental protocol. A murine model of DNCB‐induced AD was established through DNCB sensitization on day 0 as well as through repeated DNCB challenges on days 7–49 to induce AD. FFA4 WT mice were treated with either PBS or DNCB, and compound A (an FFA4 agonist) was administered by i.p. injection 30 min before DNCB challenge. b Macroscopic view of the ears. c H&E staining of ear tissue sections. d Toluidine blue staining of ear samples. e The number of mast cells (dark violet‐stained cells) was counted using a light microscope. f Blood was collected on day 49 of the experiment. Serum levels of IgE were measured by ELISA. The results are presented as the means ± SEM (n = 5). ***P < 0.001 vs. the vehicle-treated group, ###P < 0.001, ##P < 0.01 vs. the DNCB-treated group.
Histologic analysis and mast cell count in the skin
After sacrifice on day 49, ear sections from mice from the different experimental groups were prepared and examined. Briefly, the ears were fixed in 10% formalin, dehydrated in 30% sucrose solution and embedded in O.C.T. compound. The sections (8 μm) were stained with toluidine blue O (cat. T3260, Sigma-Aldrich, St. Louis, MO, USA) to identify the mast cells in the skin, and hematoxylin and eosin (H&E) staining was performed to confirm immune cell infiltration. Ear tissues from all five mice in each group were examined. For toluidine blue O staining, the O.C.T. compound was removed from the sections; the sections were rehydrated and stained with toluidine blue O staining reagent for 2 min, which was followed by rinsing, dehydration, and mounting of the sections on the coverslips. For H&E staining, the O.C.T. compound was removed from the sections, and the sections were rehydrated and stained with hematoxylin reagent for 15 s. After rinsing them with warm running tap water, the sections were stained with eosin regent for 10 s, which was followed by rinsing, dehydration, and mounting of the sections on the coverslips.
Measurement of total serum IgE levels
The immunoglobulin E levels in serum obtained from five mice per group were measured by using enzyme-linked immunosorbent assay (ELISA) kits. Briefly, 96-well plates (NUNC, Penfield, NY, USA) were coated overnight at 4 °C with a capture antibody specific for IgE (cat. 88-50460-88, eBioscience, San Diego, CA, USA). After washing, the plates were blocked with a blocking buffer for 2 h at room temperature. Serial dilutions of standard IgE were prepared and added to the appropriate wells; alternatively, serum was added to the wells. The plates were incubated for 2 h at room temperature with shaking and then washed two times. Biotinylated detection antibody specific for IgE (cat. 88-50460-88, eBioscience) was then added, and the plates were incubated for 1 h at room temperature with shaking. Then, the plates were washed four times, and avidin-horseradish peroxidase was added and incubated at room temperature for 30 min with shaking. The plates were then washed four times and incubated with the tetramethylbenzidine substrate solution at room temperature for 15 min. Then, the stop solution was added, and the absorbance of the final product was measured at 450 nm.
Flow cytometry
To determine the CD4+Foxp3+ cell population in the lymph nodes, single cells isolated from the lymph nodes were first stained at 4 °C for 15 min with a FITC-labeled rat antibody against CD4 (cat. 11-0041-82, eBioscience), which is a cell surface marker. Cells were fixed at room temperature for 1 h by using IC fixation buffer (cat. 00-8222-49, eBioscience). After cell fixation, the cells were permeabilized with permeabilization buffer (cat. 88-8824-00, eBioscience) and then stained at room temperature for 1 h with APC-labeled rat anti-Foxp3 (cat. 17-5773-82, eBioscience).
Immunofluorescence staining
Lymph nodes were fixed in 10% formalin and dehydrated in 30% sucrose solution for 24 h at 4 °C. The tissue samples were then embedded in O.C.T. compound. Sections (8 μm) were washed with PBS-T (PBS containing 0.5% Tween-20) and permeabilized with permeabilization buffer (PBS containing 0.5% Triton X-100) for 30 min at room temperature. Then, the sections were blocked with blocking solution (PBS-T containing 5% bovine serum albumin) for 30 min at room temperature. After blocking, the sections were labeled with antibodies against rat anti-Foxp3 (17-5773-82, 1:100, eBioscience) for 1 h at room temperature. Counterstaining was performed with DAPI (cat. D9542, Sigma-Aldrich) for 10 min at 37 °C. The lymph nodes were coverslipped with Vectashield mounting medium (H-1000; Vector). Immunofluorescence images were acquired using an FV10i fluorescence confocal microscope (Olympus, Tokyo, Japan).
Reverse transcription-PCR
To assess the expression levels of inflammatory markers in the lymph nodes and ears of mice by RT-PCR, the first-strand cDNA was first synthesized from total RNA isolated using TRIzol reagent (Invitrogen, Waltham, MA, USA). Total RNA was isolated from lymph nodes and ear tissues. The synthesized cDNA products, primers for each gene, and Promega Go-Taq DNA polymerase (Madison, WI, USA) were used for PCR. The specific primers and PCR conditions used were previously described [22]. Aliquots (7 μL) were electrophoresed in 1.2% agarose gels and stained with StaySafeTM Nucleic Acid Gel Stain (Real Biotech Corporation, Taipei, Taiwan). The intensities of each PCR product were quantified by using ImageJ software (NIH, Bethesda, MD, USA) and normalized to the levels of GAPDH.
Statistical analysis
The results are expressed as the means ± standard error of the mean (SEM) of five measurements for animal experiments. The statistical significance of the differences was determined by analysis of variance and Tukey’s multiple comparison test. Statistical significance was indicated by P values < 0.05. Asterisk (*) indicates a significant difference compared with the vehicle-treated group; hash (#) indicates a significant difference compared with the DNCB-treated group. The analyses were performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Compound A suppressed DNCB-induced AD in the ears of FFA4 WT mice
To investigate the roles of FFA4, we used an in vivo DNCB-induced AD model in FFA4 WT BALB/c mice and treated mice with compound A in the elicitation phase to demonstrate the therapeutic potential of FFA4. Mice (n = 5 per group) were sensitized by topical application of 1% DNCB on shaved ventral skin. After 7 days, the mice were treated with 0.3% DNCB on both ears for 42 days every other day to induce AD (Fig. 1a). Compound A (30 mg/kg) was injected intraperitoneally 30 min before DNCB challenge starting on Day 19 (Fig. 1a). The symptoms of AD are characterized by edema, erythema, and cracking of the skin area. Compared with the control group, the DNCB group showed significant increases in AD symptoms such as erythema and edema of the ear tissues (Fig. 1b). Mice treated with DNCB showed more excessive infiltration of immune cells and thickening of the epidermis due to hyperkeratosis than the control group by H&E staining (Fig. 1c). Extensive DNCB-induced hypertrophy of the epidermis and immune infiltration in the ears were significantly suppressed by compound A treatment in the FFA4 WT mice (Fig. 1c). Infiltration of mast cells into the dermis was measured by toluidine blue O staining. In AD skin lesions, mast cells accumulate, and release histamine, causing itching [23]. The mast cells appear as small, red-purple dots (Fig. 1d). However, the sections from the mice in the DNCB plus compound A-treated group had fewer stained cells than sections from mice in the DNCB group (Fig. 1d). Furthermore, the infiltration of mast cells was semiquantitatively analyzed by counting the number of mast cells in the ears. The number of mast cells was increased in the DNCB group compared with that in the control group, but the increased infiltration of mast cells into the dermis was suppressed by compound A treatment, as shown in Fig. 1e. Serum IgE levels were measured to examine the immunological effect of DNCB. Patients with AD commonly have elevated total IgE levels, and the level of IgE appears to be correlated with disease severity [24]. Hyperproduction of IgE was observed in DNCB-treated mice, and compound A treatment suppressed the elevation of serum IgE levels in FFA4 WT mice (Fig. 1f).
The levels of the inflammatory cytokines IL-4, IL-13, IL-17A, and INF-γ were also measured in the ears because AD is considered to be regulated not only by the Th2 response but also by the Th1 and Th17 responses [25,26,27]. The mRNA levels of four cytokines were significantly elevated in the ear tissues after AD induction in FFA4 WT mice (Fig. 2). The increases in the levels of the inflammatory cytokines IL-4, IL-13, IL-17, and INF-γ in the ears were significantly reduced by compound A treatment in FFA4 WT mice (Fig. 2).
Analysis of the mRNA expression of Th2 (IL-4 and IL-13), Th1 (IFN-γ), and Th17 (IL-17A) cytokines in ear tissues from the DNCB-induced and compound A-treated FFA4 WT groups. a–d The relative mRNA levels of cytokines were quantified by determining the ratios of their levels to GAPDH transcript levels. e Representative RT-PCR images are shown. The results are presented as the means ± SEM (n = 5). ***P < 0.001 vs. the vehicle-treated group, ###P < 0.001, ##P < 0.01, #P < 0.05 vs. the DNCB-treated group.
Compound A suppressed DNCB-induced atopic responses in the lymph nodes of FFA4 WT mice
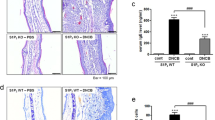
Next, we checked the lymph node size and cytokine production in the cervical lymph nodes because the cervical lymph nodes are closest to the ears. The lymph node is a part of the lymphatic system and is enclosed by a capsule. It is filled with lymphocytes containing B cells and T cells and is characterized by swelling when an infection or an allergic reaction occurs. In the AD mouse group induced by DNCB, the lymph nodes were much more swollen than those in the control group, and the lymph node weights were increased (Fig. 3a, b). Compound A significantly suppressed the DNCB-induced increase in lymph node weight by 57.1% in WT mice (Fig. 3a, b). The levels of the inflammatory cytokines IL-4, IL-13, IL-17A, and INF-γ were also measured in the lymph nodes. The mRNA levels of these four cytokines were significantly elevated in the lymph nodes after AD induction in WT mice (Fig. 3c–f). Compound A treatment suppressed the elevation of the four cytokine levels (Fig. 3c–f).
a The cervical lymph nodes were photographed to record morphological changes. b Weights of lymph nodes that were measured. c–f RT-PCR analyses of Th2 cytokines (IL-4 and IL-13), Th17 cytokines (IL-17A), and Th1 cytokines (INF-γ) were performed using mRNA isolated from cervical lymph node tissues. mRNA levels were also expressed as the ratios versus the levels of GAPDH mRNA. The results are presented as the means ± SEM (n = 5). ***P < 0.001, *P < 0.05 vs. the vehicle-treated group, ###P < 0.001, ##P < 0.01 vs. the DNCB-treated group.
Regulatory T cells are a subpopulation of T cells that are important for regulating immune responses to maintain homeostasis and self-tolerance. Previously, the beneficial effect of DHA supplementation on the development of AD was found to be mediated through M2 macrophage polarization and CD4+Foxp3+ regulatory T-cell generation [8]. Because FFA4-mediated M2 macrophage induction was caused by compound A in vivo [10, 15], we measured the numbers of CD4+Foxp3+ regulatory T cells in the lymph nodes. We isolated the cells of the cervical lymph nodes and examined the changes in CD4+Foxp3+ cells by immunofluorescent staining and FACS analysis. Immunofluorescent staining of lymph node sections showed that the number of Foxp3+-stained cells was increased in the DNCB plus compound A-treated group compared with that in the DNCB group (Fig. 4a). According to FACS analysis, DNCB did not induce any change in the CD4+Foxp3+ regulatory T-cell numbers, but compound A increased the number of CD4+Foxp3+ regulatory T-cell numbers (Fig. 4b, c).
a Lymph nodes of FFA4 WT mice with DNCB-induced AD were labeled with rat anti-Foxp3 (green), and counterstaining was conducted using DAPI (blue). b FACS analysis of CD4+Foxp3+ T cells. c Representative flow cytometry dot plots. The results are presented as the means ± SEM (n = 5). #P < 0.05 vs. the DNCB-treated group.
Compound A did not suppress DNCB-induced AD in the ears of FFA4 KO mice
To verify the involvement of FFA4 in compound A efficacy, we conducted the same experiment in FFA4 KO BALB/c mice (Fig. 5). Compared with those in the control group, AD symptoms such as erythema and edema of the ear tissues were significantly increased in the DNCB group (Fig. 5a). DNCB-induced AD in the ears of FFA4 KO mice, which was confirmed by the extensive hypertrophy of the epidermis revealed by H&E staining than that revealed in the vehicle-treated control group (Fig. 5b). Extensive DNCB-induced hypertrophy of the epidermis on the ears was not suppressed by compound A treatment in FFA4 KO mice (Fig. 5b). Toluidine blue O staining showed that the number of mast cells was significantly increased by DNCB treatment in FFA4 KO mice (Fig. 5c, d). However, the increased infiltration of mast cells into the dermis was not suppressed by compound A treatment, as shown in Fig. 5c, d. In FFA4 KO mice, hyperproduction of IgE was observed in DNCB-treated mice, but compound A treatment did not suppress the elevation of serum IgE levels (Fig. 5e). The levels of the inflammatory cytokines IL-4, IL-13, IL-17A, and INF-γ were also significantly elevated in the ear tissues after AD induction in FFA4 KO mice but were not reduced by compound A treatment in FFA4 KO mice (Fig. 6).
A murine model of DNCB‐induced AD was generated in FFA4 KO mice by DNCB sensitization on day 0 as well as through repeated DNCB challenges on days 7–49 to induce AD. FFA4 KO mice were treated with either PBS or DNCB, and compound A (an FFA4 agonist) was administered by i.p. injection 30 min before DNCB challenge. a Macroscopic view of the ears. b H&E staining of ear tissue sections. c Toluidine blue staining of ear samples. d The number of mast cells (dark violet‐stained cells) was counted using a light microscope. e Blood was collected on day 49 of the experiment. The serum levels of IgE were measured by ELISA. The results are presented as the means ± SEM (n = 5). ***P < 0.001 vs. the vehicle-treated group.
Analysis of the mRNA expression of Th2 (IL-4 and IL-13), Th1 (IFN-γ), and Th17 (IL-17A) cytokines in ear tissues from the DNCB-induced and compound A-treated FFA4 KO mice groups. a–d Relative mRNA levels of cytokines were quantified by determining the ratios of their levels to GAPDH transcript levels. e Representative RT-PCR images are shown. The results are presented as the means ± SEM (n = 5). ***P < 0.001, **P < 0.01 vs. the vehicle-treated group.
Compound A did not suppress DNCB-induced atopic responses in the lymph nodes of FFA4 KO mice
DNCB induced an increase in the sizes of the draining lymph nodes; however, the increase was not suppressed by treatment with compound A in FFA4 KO mice (Fig. 7a, b). The levels of the inflammatory cytokines IL-4, IL-13, IL-17A, and INF-γ were also measured in the lymph nodes. The mRNA levels of the four cytokines were elevated in the lymph nodes after AD induction in FFA4 KO mice but were not reduced by compound A treatment (Fig. 7c–f). In addition, we measured the numbers of CD4+Foxp3+ regulatory T cells in the lymph nodes. Compound A did not increase the numbers of CD4+Foxp3+ regulatory T cells in FFA4 KO mice (Fig. 8).
a The cervical lymph nodes were photographed to record morphological changes. b The weights of the lymph nodes were measured. c–f RT-PCR analyses of Th2 cytokines (IL-4 and IL-13), Th17 cytokines (IL-17A), and Th1 cytokines (INF-γ) were performed using mRNA isolated from cervical lymph node tissues. mRNA levels were also expressed as ratios versus the level of GAPDH mRNA. The results are presented as the means ± SEM (n = 5). ***P < 0.001, *P < 0.05 vs. the vehicle-treated group.
Discussion
As a dietary intervention, supplementation with omega-3 polyunsaturated fatty acids has been studied in AD, but many trials had unconvincing results, as no significant effect of supplementation was found [28]. This may be due to the use of low doses of omega-3 polyunsaturated fatty acids because high amounts of DHA supplementation (5.4 g per day) resulted in the improvement in Scoring AD scores in patients [7]. Similarly, in a mouse experiment, high-dosage DHA supplementation (100 mg· kg−1· d−1) suppressed the development of AD [8]. However, supplementation with high doses of omega-3 fatty acids could lead to side effects such as gastrointestinal discomfort, and the therapeutic dose needed in both studies exceeded 90–450 mg DHA, which is the standard dose for open label use of omega-3 fatty acid supplements [29, 30]. Therefore, determining the therapeutic target of omega-3 fatty acids could provide a basis for the development of a highly potent and novel medicine for AD that does not have side effects. In this study, we identified FFA4 as a therapeutic target for AD by using a selective FFA4 agonist, compound A, and FFA4-deficient mice [15,16,17, 20, 21]. Treatment with compound A not only ameliorated AD but also increased the number of CD4+Foxp3+ regulatory T cells, as was observed for treatment with DHA in FFA4 WT mice [8]. Regulatory T cells regulate excessive immune responses implicated in allergic conditions such as AD, food allergies, and asthma. High levels of serum IgE, induction of Th1 and Th2 cytokines, and allergic inflammation in the skin of regulatory T-cell-deficient mice were observed [31]. The regulatory T-cell percentage and Foxp3 mRNA level were decreased in the peripheral blood of AD patients compared with those in healthy donors, which may affect the severity of the disease [32]. Therefore, it is assumed that the activation of FFA4 by compound A inhibited the action of pro-inflammatory effector T cells (Th1, Th2, and Th17) by generating immunosuppressive regulatory T cells. The hypothesized mechanism of action of FFA4 is shown in Fig. 9, which is supported by three previous findings and the findings of the present report [8, 10, 15]. Supplementation with DHA suppressed the development of AD, and the underlying mechanism was shown to involve M2 macrophage polarization by DHA and subsequent M2 macrophage-mediated increases in CD4+Foxp3+ regulatory T-cell generation [8]. DHA and compound A-induced M2 macrophage polarization in adipose tissues in vivo in an FFA4-dependent manner [10, 15]. We showed that compound A increased CD4+Foxp3+ regulatory T cells in the lymph nodes compared with DNCB group and suppressed the development of AD in an FFA4-dependent manner.
On the other hand, Wannick et al. reported that oral administration of 50 mg/kg compound A was not effective in alleviating tissue inflammation in mouse models of autoimmune diseases, specifically imiquimod-induced psoriasis-like dermatitis, K/BxN serum transfer arthritis, and antibody transfer pemphigoid disease-like dermatitis; the dosage of compound A used in this study was even higher than that used in our study (30 mg/kg) [33]. There are three different factors to consider when comparing the two studies. One is the mouse strains that were used (C57BL/6 and BALB/c). The second is the administration routes that were used (oral administration and intraperitoneal injection). The third is the experimental models that were used (autoimmune diseases and DNCB-induced AD). Because compound A was effective against insulin resistance and adipose tissue inflammation in C57BL6 mice when administered orally [15], two factors could not explain the negative outcomes of the autoimmune disease models used by Wannick et al. Further investigation is necessary to elucidate why compound A was not effective against autoimmune diseases. Wannick et al. suggested that FFA4 activation does not significantly contribute to the health-promoting effects of omega-3 polyunsaturated fatty acids in autoimmune diseases [33]. This possibility could be partly supported by the specialized proresolving mediators of DHA and EPA derivatives, such as resolvin D1, resolvin D2, resolvin E, protectin D1, and maresin 1 [34]. Their corresponding G protein-coupled receptors (GPCRs), such as GPR32, GPR18, ChemR23, GPR37, and LGR6, have been found to function as anti-inflammatory and proresolving receptors [34,35,36,37,38,39,40]. Therefore, the beneficial effects of omega-3 polyunsaturated fatty acids may partially result from the direct activation of FFA4 and partially from the indirect activation of the GPCRs of specialized proresolving mediators [34,35,36,37,38,39,40]. Because compound A still has a low affinity for FFA4, we used a high dose of compound A (30 mg/kg). However, in the future, an FFA4 agonist with better pharmacodynamic and pharmacokinetic characteristics would represent a new therapeutic method for the treatment of AD by generating regulatory T cells.
References
Davidson WF, Leung DYM, Beck LA, Berin CM, Boguniewicz M, Busse WW, et al. Report from the national institute of allergy and infectious diseases workshop on “atopic dermatitis and the atopic march: mechanisms and interventions”. J Allergy Clin Immunol. 2019;143:894–913.
Leung DY, Guttman-Yassky E. Assessing the current treatment of atopic dermatitis: unmet needs. J Allergy Clin Immunol. 2017;139:S47–8.
Novak N. An update on the role of human dendritic cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129:879–86.
Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:336–49.
Im DS. FFA4 (GPR120) as a fatty acid sensor involved in appetite control, insulin sensitivity and inflammation regulation. Mol Asp Med. 2018;64:92–108.
Ishihara T, Yoshida M, Arita M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int Immunol. 2019;31:559–67.
Koch C, Dolle S, Metzger M, Rasche C, Jungclas H, Ruhl R, et al. Docosahexaenoic acid (DHA) supplementation in atopic eczema: a randomized, double-blind, controlled trial. Br J Dermatol. 2008;158:786–92.
Han SC, Koo DH, Kang NJ, Yoon WJ, Kang GJ, Kang HK, et al. Docosahexaenoic acid alleviates atopic dermatitis by generating Tregs and IL-10/TGF-beta-modified macrophages via a TGF-beta-dependent mechanism. J Investig Dermatol. 2015;135:1556–64.
Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–4.
Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98.
Talukdar S, Olefsky JM, Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharm Sci. 2011;32:543–50.
Moniri NH. Free-fatty acid receptor-4 (GPR120): cellular and molecular function and its role in metabolic disorders. Biochem Pharmacol. 2016;110–111:1–15.
Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159:318–32.
Konno Y, Ueki S, Takeda M, Kobayashi Y, Tamaki M, Moritoki Y, et al. Functional analysis of free fatty acid receptor GPR120 in human eosinophils: implications in metabolic homeostasis. PLoS One. 2015;10:e0120386.
Oh DY, Walenta E, Akiyama TE, Lagakos WS, Lackey D, Pessentheiner AR, et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med. 2014;20:942–7.
Lee KP, Park SJ, Kang S, Koh JM, Sato K, Chung HY, et al. ω-3 Polyunsaturated fatty acids accelerate airway repair by activating FFA4 in club cells. Am J Physiol Lung Cell Mol Physiol. 2017;312:L835–44.
Kang S, Huang J, Lee BK, Jung YS, Im E, Koh JM, et al. Omega-3 polyunsaturated fatty acids protect human hepatoma cells from developing steatosis through FFA4 (GPR120). Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:105–16.
Cox JM, Chu HD, Chelliah MV, Debenham JS, Eagen K, Lan P, et al. Design, synthesis, and evaluation of novel and selective G-protein coupled receptor 120 (GPR120) spirocyclic agonists. ACS Med Chem Lett. 2017;8:49–54.
Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–16.
Ahn SH, Park SY, Baek JE, Lee SY, Baek WY, Lee SY, et al. Free fatty acid receptor 4 (GPR120) stimulates bone formation and suppresses bone resorption in the presence of elevated n-3 fatty acid levels. Endocrinology. 2016;157:2621–35.
Cho HJ, Ahn SH, Lee YS, Lee SH, Im DS, Kim I, et al. Free fatty acid receptor 4 mediates the beneficial effects of n-3 fatty acids on body composition in mice. Calcif Tissue Int. 2017;101:654–62.
Park SJ, Im DS. Blockage of sphingosine-1-phosphate receptor 2 attenuates allergic asthma in mice. Br J Pharmacol. 2019;176:938–49.
Voisin T, Chiu IM. Molecular link between itch and atopic dermatitis. Proc Natl Acad Sci USA. 2018;115:12851–3.
Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis? J Allergy Clin Immunol. 2004;114:150–8.
Kim JY, Jeong MS, Park MK, Lee MK, Seo SJ. Time-dependent progression from the acute to chronic phases in atopic dermatitis induced by epicutaneous allergen stimulation in NC/Nga mice. Exp Dermatol. 2014;23:53–7.
Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Investig Dermatol. 2008;128:2625–30.
Muraro A, Lemanske RF Jr., Hellings PW, Akdis CA, Bieber T, Casale TB, et al. Precision medicine in patients with allergic diseases: airway diseases and atopic dermatitis-PRACTALL document of the European academy of allergy and clinical immunology and the American academy of allergy, asthma & immunology. J Allergy Clin Immunol. 2016;137:1347–58.
Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. 2019;58:1371–6.
Haitz KA, Anandasabapathy N. Docosahexaenoic acid alleviates atopic dermatitis in mice by generating T regulatory cells and m2 macrophages. J Investig Dermatol. 2015;135:1472–4.
Laidlaw M, Cockerline CA, Rowe WJ. A randomized clinical trial to determine the efficacy of manufacturers' recommended doses of omega-3 fatty acids from different sources in facilitating cardiovascular disease risk reduction. Lipids Health Dis. 2014;13:99.
Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, et al. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol. 2005;116:1106–15.
Ma L, Xue HB, Guan XH, Shu CM, Wang F, Zhang JH, et al. The imbalance of Th17 cells and CD4+ CD25high Foxp3+ Treg cells in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2014;28:1079–86.
Wannick M, Bezdek S, Guillen N, Thieme M, Meshrkey F, Mousavi S, et al. Oral administration of the selective GPR120/FFA4 agonist compound A is not effective in alleviating tissue inflammation in mouse models of prototypical autoimmune diseases. Pharmacol Res Perspect. 2018;6:e00438.
Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Asp Med. 2017;58:114–29.
Im DS. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog Lipid Res. 2012;51:232–7.
Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–22.
Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci USA. 2010;107:1660–5.
Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212:1203–17.
Qu L, Caterina MJ. Accelerating the reversal of inflammatory pain with NPD1 and its receptor GPR37. J Clin Investig. 2018;128:3246–9.
Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Investig. 2019;129:5294–311.
Acknowledgements
This research was supported by the Basic Science Research Program of the Korean National Research Foundation, which is funded by the Korean Ministry of Education, Science and Technology (NRF-2019R1A2C1005523).
Author information
Authors and Affiliations
Contributions
SES, JMK, and DSI designed the experiments. SES and SJP performed the experiments and analyzed the data. SES and DSI wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Son, SE., Park, SJ., Koh, JM. et al. Free fatty acid receptor 4 (FFA4) activation ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis by increasing regulatory T cells in mice. Acta Pharmacol Sin 41, 1337–1347 (2020). https://doi.org/10.1038/s41401-020-0435-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-0435-1
Keywords
This article is cited by
-
Inhibitory Effect of Oroxylin A in a Mouse Model of Atopic Dermatitis
Inflammation (2023)