Abstract
Study design
Retrospective study.
Objectives
We aimed to characterize the convergent disruptions of the structural connectivity based on network modeling technique (i.e., graph theory) to identify significant changes in network organization/reorganization between uninjured and chronic spinal cord injury (SCI) participants.
Setting
USA.
Methods
Ten adult participants including 4 with chronic SCI and 6 uninjured were scanned using a multi-shell diffusion imaging on a 3.0 T MR scanner. Whole brain structural connectivity matrix was estimated by performing the quantification of the number of white matter fibers (called edges) connecting each possible pair of brain region (called nodes). Brain regions were defined according to Desikan–Killiany cortical atlas. Using connectivity matrix, connectivity strength as well as six different graph theoretical measurements were computed for each participant. They include: (1) global efficiency; (2) local efficiency; (3) degree; (4) betweenness centrality; (5) average shortest length and (6) clustering coefficient. Finally network based statistics was applied to extract nodes/connections with significant differences between groups (uninjured vs SCI).
Results
The SCI group showed significant decreases in betweenness centrality in the left precentral gyrus (T-score=2.98, p value=0.02), and the right caudal middle frontal gyrus (score = 2.35, p value=0.047). It also showed significant decrease in left transverse temporal gyrus (T-score=2.36, p value=0.046) in clustering coefficient. In addition, altered regions in the occipital and parietal lobe were also identified.
Conclusion
These results suggest that not only local but also global alterations of the white matter occur after SCI. The proposed modeling technique has the potential to serve as a screening tool to identify any areas of the brain affected after SCI.
Similar content being viewed by others
Introduction
An imbalance of afferent and efferent impulses is transmitted throughout the neuroaxis after a spinal cord injury (SCI), subsequently resulting in structural and functional reorganization in multiple cortical areas [1, 2]. Electrophysiological and neuroimaging studies have shown that the deafferentation caused by SCI can result in brain cortical reorganization [1]. This cortical network reorganization may play an important role in functional recovery but aberrant reorganization has both structural and functional ramifications [3]. Several studies have been conducted so far to characterize the cortical reorganization that occurs post SCI. Green et al. (1998) were able to use electroencephalography (EEG) to map motor potentials (MP) of movement-associated cortical potentials [4]. They were able to demonstrate reorganization of cortical activity more posterior to the primary sensory cortex. This was found to have a significant correlation with motor recovery and in determining prognosis in complete and incomplete SCI.
A study by Wrigley et al. (2009) used diffusion tensor imaging (DTI) to analyze anatomical brain changes post SCI [5]. They found significant decreases in DTI parameters of the primary motor and somatosensory cortices and the superior cerebellar cortex. The changes in the superior cerebellar cortex were remarkable since, unlike the primary motor and sensory cortices, the superior cerebellar cortex has most of its connections with the brainstem and cerebral cortex and not the spinal cord [5, 6]. This shows that there are structural reorganizations in the brain that result from a secondary change and not from direct injury or spinal pathway disruptions.
More recent findings have led to the hypothesis that SCI affects brain networks in the large scale that involves multiple brain regions or interconnected systems rather than the previously believed idea that it only involves specific brain network such as sensorimotor network [7, 8]. Graph theory is a mathematical framework that can be used to map out different patterns of structural and functional connectivity of brain regions in a noninvasive manner [9]. First used by Leonhard Euler to solve a mathematical problem involving bridges in a city called Kaliningrad, this approach has been extended to various fields of studies including physics, genetics and statistics [10]. In recent years, graph theory has been gaining popularity in studies involving different aspects of brain connectivity and pathologies associated with it including epilepsy, Parkinson’s, Alzheimer’s and schizophrenia [11]. Structural connectivity represents the anatomical associations between different brain regions while functional connectivity refers to the temporal correlation of signals in various regions of the brain [12]. Kaushal et al. (2017) used graph theory to determine the functional connectivity architecture in 15 patients with chronic SCI [13]. The authors were able to generate association matrices between nodes in each individual brain and determined the presence of altered functional connectivity in various subnetworks in the SCI participants. This indicated the existence of reorganization in the cortical areas of the SCI brain [14]. Similarly, graph theory and connectomics with the help of DTI can be used to assess the presence and degree of alterations in the structural connectome in brains of chronic SCI patients.
There is a gap in literature in relation to structural connectomes in chronic SCI. In this exploratory preliminary study, we aimed to determine if there is a difference in brain structural connectomes of uninjured participants in comparison to persons with chronic SCI using diffusion tensor tractography and graph theory. We also examined if there were specific alterations to the structural connectome of participants with chronic SCI. We hypothesize that connectome analysis of the brain will reveal structural variations between injured chronic SCI and uninjured groups. To the best of our knowledge, this is the first study, with the help of graph theory, to assess structural connectome alterations in the brains of persons with chronic SCI as compared to uninjured populations.
Methods
The study was approved by the institutional review board (IRB) of Thomas Jefferson University Hospital. All methods were performed in accordance with the relevant guidelines and regulations approved by IRB. Informed consent was obtained from all patients. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research
Participants
A total of 10 participants including 4 (mean 46.38 (SD 18.67) years) with chronic SCI and 6 neurologically intact controls (mean 25.16 (SD 5.60) years) were recruited and scanned on a 3.0 T Ingenia Philips scanner equipped with 32 channel head coil. Participants signed a written informed consent document approved by the governing IRB. As part of clinical procedure, all the participants with SCI underwent a full neurological evaluation based on International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) to define level of injury and American Spinal Injury Association (ASIA) Impairment Scale (AIS) to determine severity of injury (Table 1). As per inclusion criteria for persons with chronic SCI recruitment, participants had no neurological change in the past 3 months prior to neuroimaging visit. Recruited SCI participants had no history of neurological diseases or disorders unrelated to the SCI.
Imaging
The MR Imaging protocol was included: (1) High resolution three dimensional (3D) T1-weighted anatomic scan based on magnetization-prepared-rapid-acquisition-gradient-echo (MPRAGE) sequence (TR/TE/α = 7.0 ms/3.0 ms/9°, 1.0mm3 isotropic voxels, axial slices=180, slice thickness=1 mm thickness, matrix size=512×512, FOV = 25 cm, NEX = 1) and (2) Multi-shell diffusion imaging using single shot echo planar imaging (EPI) sequence (~15 min, b-values = 800 and 2800 sec/mm2, a total of 109 diffusion-weighting gradient directions (30 and 60 in each shell) were encoded, TR/TE/α = 6.4 s/120 ms/90°, 2.5 mm3 isotropic voxels, axial slices = 45, matrix size=128×128, FOV = 24 cm, number of averages = 1 (with 19 b0 images)).
Data preprocessing
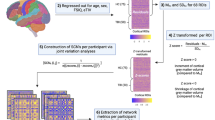
Initially, partial volume fraction maps of white matter (WM), cortical gray matter (GM), deep GM, and cerebrospinal fluid (CSF), were calculated from the T1W data. Using partial volume maps, GM–WM interface mask was obtained using the “5tt2gmwmi” command, which was implemented in the MRtrix (http://www.mrtrix.org/) and then used to process for the multi-tissue (MT)-constrained spherical deconvolution (CSD) and anatomically-constrained tractography (ACT) framework. Next, T1-weighted data from every participant was co-registered to dMRI space (average b0) using a rigid body registration algorithm with 6 degrees of freedom of FSL FLIRT toolbox (http://www.fmrib.ox.ac.uk/fsl). The estimated rigid transformation matrix was then applied to both segmentation and parcellation data computed in freesurfer (https://surfer.nmr.mgh.harvard.edu/). The regions extracted from the parcellation map consisted of 84 cortical and subcortical regions (Table 2). To construct whole brain fiber tracts, first high angular resolution diffusion imaging (HARDI) acquisition (outer shell) was separated from multi-shell data and corrected for motion and eddy current artifacts using EDDY toolbox of FSL. Spherical deconvolution informed filtering of tractograms and ACT were applied to reduce bias in streamline density and to prevent biologically unrealistic connection terminations [15]. All diffusion-based tractography approaches and subsequent connectome reconstructions were performed in MRtrix3 (https://www.mrtrix.org/) (Fig. 1).
Connectome construction and graph theory analysis
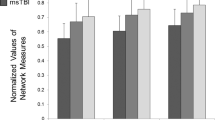
Structural connectivity maps were constructed by comparing the preprocessed data for each brain generated tracks between the 84 cortical and subcortical regions called “nodes” according to Desikan–Killiany cortical atlas segmentation (https://surfer.nmr.mgh.harvard.edu/fswiki). Two regions were considered to be structurally connected if one or more streamlines had their endpoints in both regions [16]. The number of streamlines interconnecting each pair of nodes was enumerated and called “edges”. This resulted in an 84 × 84 interregional connectivity matrix called “adjacency matrix”, with each element of this matrix (edge) represents the connectivity strength. Note that negative values and diagonal elements (self-connections) were set to zero. After construction of connectivity matrix, global and local topological measures were analyzed based on graph theory algorithm developed in GRETNA toolbox (https://www.nitrc.org/projects/gretna/) [17]. The following six network metrics as well as connectivity strength were computed for each participant and compared for statistical significance between groups using two tailed student t test. A p value less than 0.05 was considered statistically significant. Graph measures were included: (1) global efficiency; (2) local efficiency; (3) degree; (4) betweenness centrality; (5) average shortest path length and (6) clustering coefficient. These measures are most commonly used graph metrics characterizing the structural and functional brain network. Table 3 represents the basic concept of aforementioned graph measures and their definitions.
Results
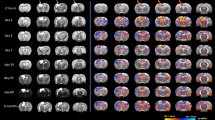
The strength of connections between every possible pair of ROIs was computed to generate association or adjacency matrix for individual participants. Figure 2 represents the averaged group adjacency matrix for SCI and uninjured participants. Qualitative visual inspection of the adjacency matrix suggests that SCI group demonstrates a different pattern of connectivity compared to uninjured group (Fig. 2). Despite the observed regional abnormalities several network graph properties were significantly different between the two groups.
Average structural connectivity matrix or adjacency matrix of uninjured (upper left) and SCI participants (upper right). ROI to ROI connectivity ring representing statistically significant connections between pair of ROIs (p value < 0.01) comparing uninjured with SCI population. Labels have identified in Table 2.
There was an elevation of strength of connections at 11 pairs of ROIs and reduction in one pair as shown in Table 4 in the SCI group. As seen in Table 4, the strongest increase in structural connectivity was obtained between temporal lobe structures that included (1) left inferior temporal gyrus and right amygdala (t value = −4.00, p value=0.004); (2) left fusiform and left temporal pole (t value = −4.21, p value=0.003); and (3) left temporal pole and right fusiform (t value = −3.79, p value=0.005). Increased structural connectivity between sensorimotor and speech articulation network were shown in SCI patients. These regions included (1) left paracentral gyrus and left caudal middle frontal gyrus (t value = −4.02, p value=0.004), (2) left paracentral and right insula (t value = −3.52, p value=0.008) and (3) left postcentral and right pars opercularis (t value = −3.43, p value=0.009). In addition, anatomically decreased or increased connections were shown between limbic and extra limbic structures such as right amygdala and left precuneus (t value = −3.52, p value=0.008); left caudate and left middle temporal gyrus (t value = +3.45, p value=0.009); right accumbens nucleus and rostral anterior cingulate (t value = −3.52, p value=0.008); right posterior cingulate and left transverse temporal gyrus (t value = −3.45, p value=0.008); and left palladium and left medial orbito frontal cortex (t value = −3.38, p value=0.01).
Furthermore, using network based statistics (graph theory), betweenness centrality of left precentral gyrus (t value = +2.98, p value=0.02) and right caudal middle frontal gyrus (t value = +2.35, p value=0.047) and clustering coefficient of left transverse temporal gyrus (t value = +2.36, p value=0.046) were decreased significantly in SCI participants (Table 4). Global and local efficiency, shortest path length and degree did not show any significant difference between groups.
Discussion
It remains unknown to what extent SCI can cause white matter reorganization of the human brain. Quantifying these reorganizations will aid in understanding the white matter changes of the brain following SCI. This could potentially contribute to the development of more focused treatment and rehabilitation guidelines.
Reorganization in acute SCI is most likely caused by latent synapses uncovered by modulation of neurotransmitters while in chronic SCI, cortical reorganization is caused by axonal regeneration and sprouting of collaterals, along with synaptic efficacy changes [1, 2]. An example of somatosensory reorganization was found in monkey studies by Nudo et al. (1996) and Raineteau et al. (2001) [18, 19]. They observed that cortical motor sections controlling intact body parts tend to enlarge and encroach regions that have lost their peripheral target. In a similar study, Perani et al. (2001) used fMRI in patients with SCI to demonstrate cross activation of areas representing a denervated lower limb seen when the forelimb was stimulated, which has revealed the presence of significant structural somatosensory reorganization in SCI patients [20].
Motor and sensory deficits at and below the level of the injury incite alterations in the activation pattern of old connections and sprouting of new neuronal connections which in turn evokes neuroplasticity. Somatosensory representations of sensory input from body parts are topographic. Loss of afferent inputs from a portion of the body deactivates a part of each representation on the cortex [18,19,20,21]. This study shows an increase in specific white matter tract density associated with sensorimotor networks as shown in Table 4. There was increased tract density from the left medial orbitofrontal gyrus to the left pallidum and from the left middle temporal gyrus to the left caudate. The involvement of the pallidum and the caudate indicates an involvement of the basal nuclei network in the reorganization process. Our analyses also revealed an increase in structural white matter tract density between sensorimotor areas: left paracentral lobule and left post central gyrus, auditory processing area: right insula and speech articulation area: right pars opercularis. A more in depth analysis of the consequences of these alterations could facilitate functional recovery in these patients.
In addition to the sensorimotor networks, we found significant changes in areas involved in the pain network and its perception. Pain has been reported by at least one-third of patients with SCI [22]. Nicotra et al. (2006) used fMRI to demonstrate activation of the anterior cingulate cortex during pain perception [23]. They found that in patients with peripheral autonomic denervation, during stress, there is compensatory enhancement of anterior cingulate activity. In our patient set, there was an increase in tract density from the right anterior cingulate cortex and the right accumbens, between the left postcentral gyrus and the right pars opercularis and also between the left paracentral lobule and right insula. The pain perception network includes the post central gyrus, insula, prefrontal cortex, anterior cingulate gyrus and the thalamus [24]. Our results illustrate an involvement of regions associated with the pain pathway.
Along with the sensorimotor and pain networks, we have discussed the white matter tract changes illustrated in Table 4 involving networks that are responsible for cognitive, behavioral, visual and auditory functions. Craig et al. (2017) reported that an adult with SCI has 13 times the risk of cognitive impairment when compared to healthy individuals [22]. It has been shown that up to 60% of participants with chronic SCI have deficits in attention, processing, memory and cognition [25]. The posterior cingulate gyrus is a region known to play a key function in cognition [26]. Defects in the posterior cingulate gyrus have been implicated in disorders of memory, attention and internal vs external word balance [27]. Our study results demonstrated an increase in tract density between the right anterior cingulate cortex and right accumbens area, from the left banks of the superior temporal sulcus and the left transverse temporal gyrus to the right posterior cingulate cortex. This potential evidence of neuroplasticity may have several cognitive implications. Regions affecting working memory, semantic memory and behavior such as the posterior cingulate gyrus, middle frontal gyrus, inferior temporal gyrus, left middle temporal gyrus, amygdala and the caudate have shown an increase in white matter tracts to other regions. We have demonstrated increased structural connectivity between limbic and extra limbic structures in SCI patients.
Another area of cognitive impairment that has been noted in patients with SCI is the attention and visual processing region. 41% of participants with chronic SCI showed processing speed deficits that were clinically significant [28]. Our study showed significantly increased white matter tract density in areas that are responsible for visual learning and processing such as the left inferior temporal gyrus and bilateral fusiform gyri. The implication of these specific white matter tract changes on the cognitive functioning and correlation with cognitive tests in these patients can be a subject of future studies with a larger sample size.
Graph network findings
In addition, graph theory analysis has been used to delineate topological alterations after SCI and reveal the pathophysiological mechanism of brain plasticity. In order to demonstrate the topological properties of brain network, frequently used network parameters (e.g., betweenness centrality, clustering coefficient and so on) were analyzed. In this study, betweenness centrality in the left precentral gyrus and the right caudal middle frontal gyrus were significantly lower in the SCI group. The right caudal middle frontal gyrus is located in the frontal lobe. It is a part of the prefrontal cortex and associated with executive motor functioning, word memory and retrieval [29]. The decreased betweenness centrality may be explained by the formation of possible collateral connection networks passing through these regions. The left precentral gyrus is the primary motor cortex. Functional studies have concluded that there is an altered functional connectivity in the motor regions of the sensorimotor cortex after SCI [1, 2]. Our results have shown that structurally also, this region is less connected to other nodes compared to the uninjured group.
Clustering coefficient describes the ability of brain regions to perform neural processes independently from the surrounding network through dense interconnections between them; it is a key measure of functional segregation and regularized topology in networks [30]. In our analysis, the nodal graph measures showed lower clustering coefficient of the left transverse temporal gyrus, which is involved in auditory processing and tone perception in the SCI group, suggesting a greater structural segregation with specialized interactions around these regions. This suggests that the loss of somatosensory input to the central nervous system may have an impact on cortical reactivity and subsequent cognitive task efficiency. Individuals with SCI have a permanent loss of such input to varying degrees. While this is admittedly a preliminary investigation, results are encouraging and suggest that future efforts to expand these findings would be best aimed at exploring higher order differences between the spinal cord injured and uninjured group relative to cognitive processing ability rather than more basic neurophysiologic/peripheral differences [31, 32].
In this study, there was no significant difference in participants with SCI compared to the uninjured in graph measures of local and global efficiency, degree and shortest path length. To our knowledge, there has been no previous report of the structural connectivity in SCI with graph theoretical approach. However few studies have looked at graph based functional connectivity approach using electroencephalogram (EEG) and resting state fMRI [13, 31, 32]. De Vico Fallani et al. (2007) demonstrated an increase in local efficiency but not in the global efficiency in the SCI group compared to uninjured in their motor network [33]. Using resting state fMRI, Min et al (2015) reported no significant difference in SCI compared to uninjured in clustering coefficient, global efficiency, and small-worldness. However, they found the characteristic path length to random network was higher in SCI patients [31]. In another study by Kaushal et al. (2017) local efficiency was significantly lower in SCI patients while global efficiency didn’t reach a significant level [14]. The discrepancy between current study and previous studies is due to differences between structural and functional networks.
Limitations and future work
While this study yielded several novel results, there are limitations to the study that must be taken into account when interpreting our results. Due to the small sample size, our findings must be interpreted as a preliminary and a potential guideline for future work. Second, the evaluation of graph theory findings to draw clinical conclusions presents difficulty due to ambiguity in discerning whether network changes are a consequence of injury itself or variability in the type of drugs used, duration and type of rehabilitation, therapeutic intervention or involvement of pain and traumatic brain injury. The different clinical presentations may represent different extent of involvement of the network. Therefore, the identification of differences in graph theory metrics might be biased by these clinical presentations. This limitation can be addressed in further studies by including a sample large enough to cluster patients according to the specific type of intervention and injury. Future studies may add clinical parameters in association with the connectivity parameters to assess for correlations and significance. Past studies have revealed age, gender, laterality, severity and level of injury related changes in functional and structural connectivity. In the future, we will attempt to correct for these variables however, the analysis will be powered by increasing sample size.
Conclusion
Structural connections were successfully visualized in the entire brain in both uninjured and SCI participants and the strength of the connectivities evaluated quantitatively among various areas by using diffusion tractography in this preliminary study. This method may serve as a valuable tool to reveal pathological changes in the brain after SCI. These findings may imply modular reorganization due to neural plasticity. Further analysis, such as topological rearrangement and hub region identification in conjunction with neuropsychological and cognitive processing, are needed with a larger sample size for a more robust understanding of the neuroplasticity in patients with SCI in the view of network science. In conclusion, Connectome analysis can be a valuable diagnostic and prognostic tool that can be directly applied to the clinic protocols to guide clinicians to determine the best course of treatment for each individual while defining therapeutic efficacy.
References
Nardone R, Höller Y, Sebastianelli L, Versace V, Saltuari L, Brigo F, et al. Cortical morphometric changes after spinal cord injury. Brain Res Bull. 2018;137:107–19.
Nardone R, Höller Y, Brigo F, Seidl M, Christova M, Bergmann J, et al. Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res. 2013;1504:58–73.
Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zörner B, Schneider R, et al. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci. 2009;29:12210–9.
Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical sensorimotor reorganization after spinal cord injury: an electroencephalographic study. Neurology. 1998;50:1115–21.
Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, et al. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19:224–32.
Kokotilo KJ, Eng JJ, Curt A. Reorganization and preservation of motor control of the brain in spinal cord injury: a systematic review. J Neurotrauma. 2009;26:2113–26.
Athanasiou A, Klados MA, Pandria N, Foroglou N, Kavazidi KR, Polyzoidis K, et al. A systematic review of investigations into functional brain connectivity following spinal cord injury. Front Hum Neurosci. 2017;11:1–9.
Hawasli AH, Rutlin J, Roland JL, Murphy RKJ, Song SK, Leuthardt EC, et al. Spinal cord injury disrupts resting-state networks in the human brain. J Neurotrauma. 2018;35:864–73.
He Y, Evans A. Graph theoretical modeling of brain connectivity. Curr Opin Neurol. 2010;23:341–50.
Chiang S, Haneef Z. Graph theory findings in the pathophysiology of temporal lobe epilepsy. Clin Neurophysiol. 2014;125:1295–305.
Farahani FV, Karwowski W, Lighthall NR. Application of graph theory for identifying connectivity patterns in human brain networks: a systematic review. Front Neurosci. 2019;13:1–27.
Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98.
Kaushal M, Oni-Orisan A, Chen G, Li W, Leschke J, Ward D, et al. Large-scale network analysis of whole-brain resting-state functional connectivity in spinal cord injury: a comparative study. Brain Connect. 2017;7:413–23.
Kaushal M, Oni-Orisan A, Chen G, Li W, Leschke J, Ward BD, et al. Evaluation of whole-brain resting-state functional connectivity in spinal cord injury: a large-scale network analysis using network-based statistic. J Neurotrauma. 2017b;34:1278–82.
Kamagata K, Zalesky A, Hatano T, Di Biase MA, El Samad O, Saiki S, et al. Connectome analysis with diffusion MRI in idiopathic Parkinson’s disease: evaluation using multi-shell, multi-tissue, constrained spherical deconvolution. Neuroimage Clin. 2017;17:518–29.
Sinke MRT, Otte WM, Christiaens D, Schmitt O, Leemans A, van der Toorn A, et al. Diffusion MRI-based cortical connectome reconstruction: dependency on tractography procedures and neuroanatomical characteristics. Brain Struct Funct. 2018;223:2269–85.
Wang J, Wang X, Xia M, Liao X, Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. 2015;9:1–16.
Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–9.
Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–73.
Perani D, Brunelli GA, Tettamanti M, Scifo P, Tecchio F, Rossini PM, et al. Remodelling of sensorimotor maps in paraplegia: a functional magnetic resonance imaging study after a surgical nerve transfer. Neurosci Lett. 2001;303:62–6.
Ding Y, Kastin AJ, Pan W. Neural plasticity after spinal cord injury. Curr Pharm Des. 2005;11:1441–50.
Craig A, Guest R, Tran Y, Middleton J. Cognitive impairment and mood states after spinal cord injury. J Neurotrauma. 2017;34:1156–63.
Nicotra A, Critchley HD, Mathias CJ, Dolan RJ. Emotional and autonomic consequences of spinal cord injury explored using functional brain imaging. Brain. 2006;129:718–28.
Nakata H, Sakamoto K, Kakigi R. Meditation reduces pain-related neural activity in the anterior cingulate cortex, insula, secondary somatosensory cortex, and thalamus. Front Psychol. 2014;5:1–12.
Chiaravalloti ND, Weber E, Wylie G, Dyson-Hudson T, Wecht JM. Patterns of cognitive deficits in persons with spinal cord injury as compared with both age-matched and older individuals without spinal cord injury. J Spinal Cord Med. 2020;43:88–97.
Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32.
Wecht JM, Bauman WA. Decentralized cardiovascular autonomic control and cognitive deficits in persons with spinal cord injury. J Spinal Cord Med. 2013;36:74–81.
Dowler RN, Harrington DL, Haaland KY, Swanda RM, Fee F, Fiedler K. Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. J Int Neuropsychol Soc. 1997;3:464–72.
Bartolo R, Saunders RC, Mitz AR, Averbeck BB. Dimensionality, information and learning in prefrontal cortex. PLoS Comput Biol. 2020;16:1–26.
Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–69.
Min YS, Chang Y, Park JW, Lee JM, Cha J, Yang JJ, et al. Change of brain functional connectivity in patients with spinal cord injury: graph theory based approach. Ann Rehabil Med. 2015;39:374–83.
Richards JS, Seitz MR, Eisele WA. Auditory processing in spinal cord injury: a preliminary investigation from a sensory deprivation perspective. Arch Phys Med Rehabil. 1986;67:115–7.
De Vico Fallani F, Astolfi L, Cincotti F, Mattia D, Marciani MG, Salinari S, et al. Cortical functional connectivity networks in normal and spinal cord injured patients: evaluation by graph analysis. Hum Brain Mapp. 2007;28:1334–46.
Funding
This work was supported by provost pilot clinical research grant, Thomas Jefferson University. We also acknowledge support by NIH NINDS #NS097880 (MRD) and Department of Defense, Spinal Cord Injury Research Program Award No. W81XWH-17-1-0476.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alizadeh, M., Manmatharayan, A.R., Johnston, T. et al. Graph theoretical structural connectome analysis of the brain in patients with chronic spinal cord injury: preliminary investigation. Spinal Cord Ser Cases 7, 60 (2021). https://doi.org/10.1038/s41394-021-00424-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00424-3