Abstract
Introduction
Intradural extramedullary (IDEM) metastatic disease is infrequently encountered by spine surgeons and consequently poorly understood. Discovery often corresponds with the onset of neurologic symptoms and no consensus exists regarding the importance of complete resection or anticipated postoperative outcome. We aim to elucidate treatment methodologies that exist in the literature.
Case presentation
We present a unique case of a 57-year-old male with a known history of esophageal adenocarcinoma, including brain and visceral metastases, who presented with cauda equina syndrome. An IDEM metastatic esophageal adenocarcinoma lesion was identified on advanced imaging and biopsy. This was treated operatively without return of neurologic function.
Discussion
We reviewed and summarized the existing literature. Trends are highlighted to further guide surgeons treating this unusual metastatic phenomenon.
Conclusion
Intradural metastasis is a harbinger of advanced disease with a poor prognosis regardless of the etiology of the primary lesion. There are a number of proposed mechanisms for metastatic spread with little available literature for surgeon guidance. Most authors are advocates of a palliative, decompressive approach.
Similar content being viewed by others
Introduction
IDEM is a rare entity—uncommon enough to preclude general recommendations for surgical management and an understanding of the underlying pathophysiology. A review of the literature reveals a heterogeneous group of primary malignancies that have demonstrated metastatic potential to the intradural extramedullary space, including lung, renal, thyroid, and primary bone, among others. Much of the literature is devoted to mention the number of documented cases of each primary malignant etiology, with authors often noting that their case is the only documented case, or one of few. Given the lack of guidance in our case, it was clear that a comprehensive review of how these cases are managed is what was needed. As such, we set out to identify the differences and commonalities in how patients were treated, how they performed postoperatively, and their surgeons’ theories for pathogenesis.
We begin by presenting a unique case report of esophageal adenocarcinoma metastatic to the IDEM space which is the first reported case of its kind in the literature. Esophageal carcinoma is an aggressive malignancy when discovered in its advanced stages, and it has become increasingly prevalent in past decades [1]. The survival rate for esophageal carcinoma is poor, and when metastatic disease is present, the 5-year survival rate is less than 5% [2]. Common sites of metastasis for esophageal carcinoma include lymph node, liver, lung, bone, and brain with known dissemination via contiguous spread, lymphatics, and the blood stream [3]. Although metastasis to the vertebral column occurs in ~3–5% of all cancers, it has been rarely reported in the case of a primary esophageal neoplasm [4]. When lesions of any malignancy are present in the vertebral column, intradural lesions are only seen in 6% of these cases, further emphasizing the rarity of intradural metastatic pathology in general [5,6,7].
Case presentation
A 57-year-old male with a known history esophageal carcinoma, including brain and visceral metastases, presented to our emergency department with sudden onset of bilateral lower extremity weakness and saddle anesthesia. He stated that he was ambulatory without assistive device the evening prior to his presentation and endorsed a history of deep venous thrombosis for which he was maintained on rivaroxaban. He reported a prior surgical history of gamma knife radiation for cerebral metastases. Despite the strong association of esophageal malignancy with tobacco and alcohol, the patient’s social history was negative for alcohol and tobacco.
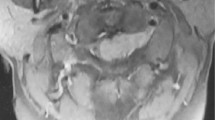
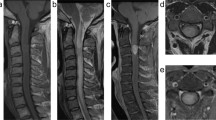
Upon examination, the patient demonstrated a normal neurologic exam in his upper extremities and abnormal exam in his lower extremities consistent with acute cauda equina syndrome. Motor power was 1/5 bilaterally at the level of L2, and 0/5 bilaterally at L3 and below. Examination also demonstrated absent sensation in the perineum, absent rectal tone, and absent reflexes. Computed tomography imaging was performed in the emergency department which identified numerous bony metastases throughout the neural axis, and no notable space occupying lesion that accounted for his symptoms of acute cauda equina syndrome. A magnetic resonance imaging study of the lumbar spine was performed which revealed an IDEM lesion at the level of the L2 vertebral body with displacement of the nerve rootlets (Figs. 1 and 2). With this imaging study completed, the diagnosis of cauda equina syndrome secondary to tumor metastasis was confirmed. A discussion about the patient’s prognosis and treatment options took place and both the primary surgeon and the patient deemed that surgery would be best treatment option to improve neurologic function.
A standard posterior approach was carried out overlying the L2 vertebral body and the sub-periosteal dissection was performed to expose the laminae of L1, L2, and L3. A complete laminectomy was performed at L2 with partial laminectomies performed at L1 and L3, as well as resection of the ligamentum flavum in order to expose the dura mater. The dura was incised longitudinally exposing the intradural lesion and rootlets. The lesion was then removed and a specimen was sent to pathology (Fig. 3). The durotomy was primarily repaired followed by the closure of the surgical surgical wound in standard fashion. Upon evaluation by pathology, the tumor was reported as a metastatic poorly differentiated non-small cell adenocarcinoma, which was consistent with the primary esophageal neoplasm.
a H&E; Patient’s primary esophageal mass–invasive moderately to poorly differentiated adenocarcinoma (×100). Note the dysplastic glands underlying the squamous epithelium. b H&E; High power view of primary esophageal mass (×400). Note the enlarged, hyperchromatic nuclei with frequent mitoses and necrosis. c H&E; Spinal intradural tumor–metastatic poorly differentiated adenocarcinoma (×100). d H&E; High power view of spinal tumor (×400). Note the similar cytological features–enlarged, hyperchromatic nuclei, increased mitoses and necrosis, to the esophageal primary
Post-operatively the patient’s bilateral lower extremity motor and sensory deficits persisted, while the overall health of the patient continued to deteriorate. Eleven days post operation the patient and the patient’s family decided to pursue palliative care. Due to the advanced stage of the esophageal carcinoma and extensive metastasis, the patient expired 2 weeks after the procedure.
Discussion and literature review
Mechanism of metastasis
The discussion regarding mechanism of metastasis for intradural lesions continues to evolve as more case series and reports are published; however, most authors propose one or a combination of 5 mechanisms for neoplastic cells reaching the IDEM space. These include spread from Batson’s venous plexus, perineural lymphatics, seeding from local invasion adjacent to the dura, spread from the sub-arachnoid space in so called “drop metastasis” or leptomeningeal fashion, and hematogenous spread via the arterial system [8]. It is unclear which route was utilized in our case, but given our patient’s known and documented intracranial metastatic lesions, we propose that the lesion was the result of spread from the sub-arachnoid space. In fact, the most commonly advocated mechanism in the literature reviewed was leptomeningeal spread [7, 9,10,11,12,13]. It follows that concurrent intracranial disease is likely to create metastatic disease given the fact that >50% of cases with an intracranial malignancy will demonstrate neoplastic cells in the CSF [9].
Kotil et al. describes a case in which an intradural metastatic lesion from small cell carcinoma of the lung presents as cauda equina syndrome [14]. As lung cancer is predisposed to spread via the hematogenous route and the authors identified no bony, visceral, or cerebral metastatic lesions, they surmised that hematogenous spread was most likely. This proposition is consistent among case reports with a known lung primary malignancy [5]. Other, more complex routes have also been described which include renal cell carcinoma spreading along the autonomic nerves to the aorticorenal, celiac, and mesenteric ganglia and then along the thoracic and lumbar splanchnic nerves to the corresponding spinal nerves to the intradural, extramedullary space [13].
Only one case was noted to mention direct invasion, because the majority of cases of intradural extramedullary metastatic disease demonstrate no extradural or bony involvement. Hematogenous spread via Batson’s plexus is an attractive proposition given the bidirectional flow and extensive network of this system—it has been implicated in a large fraction of case reports as well.
Numerous factors play roles in the routes of spread for intradural lesions and further research is warranted in identifying factors that predispose neoplasms to metastasize via specific routes to the intradural space. As an example, mutations in the (ALK) gene in non-small cell lung cancer have been shown to exhibit a propensity for CNS involvement [15]. Further advances in understanding the tropism of these neoplasms and their respective pathways for metastasis might be found in future studies exploring tumor genetics.
Surgical considerations
IDEM spinal metastasis originating from a primary tumor outside the central nervous system is exceedingly rare and usually discovered secondary to symptoms of compression of nerve root(s), the cord, conus, or cauda equine [16]. Compression of the cauda equina presents with very noticeable, predictable symptoms, and in patients with a compressive neoplastic lesion of the cauda equina, such lesions are typically extradural. Patients with an IDEM metastatic lesion involving the cauda equina have been mostly associated with primary lesions of the prostate, kidney, thyroid, lung, and breast [17]. Nonetheless, surgical treatment for intradural lesions appears to be the mainstay for tumors that present acutely with neurological deficits. As was in our case, cauda equina syndrome is universally treated surgically as it constitutes a surgical emergency.
In most cases, complete resection of the IDEM lesion seems desirable to decompress the neural elements and relieve the compressive pathology. A review of the literature, however, suggests that complete resection may be unnecessary. In the vast majority of cases, patients exhibit some subjective improvement in pain relief and or motor power post-operatively even when simple debulking or sub-total resection is performed [5,6,7, 10, 12, 14, 18,19,20,21,22,23,24,25,26]. Furthermore, it is unclear preoperatively which types of metastases will be amenable to complete resection. In some case reports, tumor resection was reported as en block [14, 19, 21, 27, 28], whereas others convey adherence of the tumor to neural elements precluding subtotal resection [7, 10, 18, 20, 22, 23, 26]. Our case was consistent with the latter—intimately associated with the intradural rootlets of the cauda equina. One author, in keeping with a palliation pain control strategy, promotes transection of the affected nerve rootlets in the event that metastatic disease is firmly adhered precluding complete resection [25].
Return of normal neurologic function, although generally improved following surgical decompression, is never guaranteed. A case report of IDEM metastatic follicular thyroid carcinoma exhibited post-operative improvement of 2/5 motor power in the affected segments to 3/5 motor power despite complete resection without intraoperative changes on neuro-monitoring [21]. Given the variable origin of these metastases, prognoses and therefore the goals of surgery are paramount in discussions with the patient preoperatively.
Adjuvant therapy
Fortunately, a number of treatment modalities exist for intradural metastatic lesions aside from surgery. Nearly every case report reviewed utilized some combination of surgery and chemotherapy or radiation therapy (RT), all of which have been described for intradural tumors with varying degrees of success. The type of primary disease that ultimately leads to intradural metastatic lesions also plays a role in treatment. Marotta et al. describe a metastatic intradural thymoma that underwent RT following surgical treatment. As is well documented in the tumor literature, this primary malignancy is radiosensitive and incomplete surgical excision is adequate given the patient’s improvement in symptoms and lesion size following a course of RT. The use of chemotherapeutics has also been employed in treatment strategies for intradural metastatic lesions [29]. Again, primary malignancy etiology and possible genetic variations are likely to play a role in whether chemotherapy is effective or not—we advocate early involvement of a multidisciplinary team including oncologic specialists in all cases of IDEM metastatic disease given its late presentation.
Prognosis
Irrespective of the etiology of the primary malignancy, the finding of IDEM metastasis portends a grim prognosis given the late nature of disease on presentation [7] and as such, most authors recommend surgery from a palliative approach [12, 21, 25, 28, 30]. Intradural metastatic lesions with a carcinoma primary are indicators of advanced disease and presence of a pre-surgical neurological deficit is an independent overall negative predictor of survival with one study reporting a hazard ratio of 10.2 [9]. Tumor morphology and physiology may also play a role in neurological recovery following intradural tumor resection. Further research is needed to identify factors of specific primary malignancies that make tumors more or less likely to be adherent to other intradural structures, as this information may have important prognostic implications.
Treatment strategies include curative, functional, and palliative and vary widely because of specific treatment goals tailored to individual patients. Much of the decision regarding surgical approach involves both the extent of neoplastic burden and the wishes of the patient. In the case report by Petterwood et al., the authors contend that despite the number of surgical options available for treatment of metastatic lesions, surgical treatment is futile for overall survival and efforts of palliation may be in the patients’ best interest in order to provide improved pain, function, and quality of life. The literature reviewed seem to suggest that pre-operative counseling for patients with intradural lesions should indicate that no significant difference in survival is to be expected, and surgery is aimed primarily at improvement in quality of life.
Conclusion
IDEM metastasis is a harbinger of advanced disease with a poor prognosis regardless of the etiology of the primary lesion. As such, patient counseling should reflect a palliative approach without anticipated improvement in survivorship with surgical efforts aimed at debulking compressive disease combined with appropriate multidisciplinary adjuvant therapy. Future research might target detection of IDEM metastasis prior to symptomatic neural compression and further investigate the genetic features that drive neoplastic tropism to the IDEM space.
References
Wu S-G, Zhang W-W, He Z-Y, Sun J-Y, Chen Y-X, Guo L. Sites of metastasis and overall survival in esophageal cancer: a population-based study. Cancer Manag Res. 2017;9:781–8. https://doi.org/10.2147/CMAR.S150350.
Tanaka T, Fujita H, Matono S, Nagano T, Nishimura K, Murata K, Shirouzu K, Suzuki G, Hayabuchi N. Yamana. Outcomes of multimodality therapy for stage IVB esophageal cancer with distant organ metastasis (M1-Org). Dis Esophagus. 2010;23:646–51.
Viaro AL, Roballo CA, de Campos PT, Teixeira CO, Teixeira MA. Occult esophageal squamous cell carcinoma with metastases to the spine and central nervous system. Autops Case Rep. 2015;5:33.
NICE Clinical Guidelines. No. 75. Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression. National Collaborating Centre for Cancer (UK). Cardiff (UK): National Collaborating Centre for Cancer (UK); 2008.
Xiong J, Zhang P. Cauda equina syndrome caused by isolated spinal extramedullaryintradural cauda equina metastasis is the primary symptom of small cell lung cancer: a case report and review of the literatrure. Int J Clin Exp Med. 2015;8:10044–50.
Lin TK, Chen SM, Jung SM. Solitary intradural extramedullary metastasis of renal cell carcinoma to the conus medullaris. Kaohsiung J Med Sci. 2011;27:45–8.
Cho SH, Rhim SC, Hyun SJ, Bae CW, Khang SK. Intradural involvement of multicentric myxoid liposarcoma. J Korean Neurosurg Soc. 2010;48:276.
Mosdal C, Bang F. Intradural spinal metastases. Acta Neurochir. 1981;56:107–10.
Knafo S, Pallud J, Le Rhun E, Parker F, Iakovlev G, Roux FX, Page P, Meder JF, Emery E, Devaux B. Club de Neuro-oncologie of the Société Française de Neurochirurgie. Intradural extramedullary spinal metastases of non-neurogenic origin: a distinct clinical entity or a subtype of leptomeningeal metastasis? A case-control study. Neurosurgery. 2013;73:923–32.
Lee CH, Kim KJ, Hyun SJ, Jahng TA, Kim HJ. Intradural extramedullary metastasis of small cell lung cancer: a case report. Korean J Spine. 2012;9:293.
Munshey A, Moore J, Maclean C, Longano A, Goldschlager T. Cranial pilocytic astrocytoma with spinal drop metastasis in an adult: case report and literature review. World Neurosurg. 2017;98:883–e7.
Stein AA, Weinstein GR, Niezgoda C, Chowdhary S, Vrionis F, Houten JK. Myelopathy from intradural extramedullary metastasis as an initial presentation of metastatic melanoma. Cureus. 2018;10:e2668.
Capek S, Krauss WE, Amrami KK, Parisi JE, Spinner RJ. Perineural spread of renal cell carcinoma: a case illustration with a proposed anatomic mechanism and a review of the literature. World Neurosurg. 2016;89:728–e11.
Kotil K, Kilinc BM, Bilge T. Spinal metastasis of occult lung carcinoma causing cauda equina syndrome. J Clin Neurosci. 2007;14:372–5.
Karsy M, Guan J, Sivakumar W, Neil JA, Schmidt MH, Mahan MA. The genetic basis of intradural spinal tumors and its impact on clinical treatment. Neurosurg focus. 2015;39.2:E3.
Perrin RG, Livingston KE, Aarabi B. Intradural extramedullary spinal metastasis: a report of 10 cases. Neurosurg. 1982;56:835–7.
Babu M, Gupta R, Kumar A, et al. Spinal intradural metastasis. JK Sci. 2004;6:48–50.
Arnold PM, Ratnasingam D, O’Neil MF, Johnson PL. Pituitary carcinoma recurrent to the lumbar intradural extramedullary space: case report. J Spinal Cord Med. 2012;35:118–21.
Gaetani P, Di Ieva A, Colombo P, Tancioni F, Aimar E, Debernardi A, Rodriguez y Baena R. Intradural spinal metastasis of renal clear cell carcinoma causing cauda equina syndrome. Acta Neurochir. 2004;146:857–61.
Haresh KP, Chinikkatti SK, Prabhakar R, Rishi A, Rath GK, Sharma DN, Julka PK. A rare case of intradural extramedullary Ewing’s sarcoma with skip metastasis in the spine. Spinal Cord. 2008;46:582.
Keen J, Milosavljevic E, Hanna G, Gospodarev V, Raghavan R, Ghostine S. Rare intradural cervical nerve root metastasis of follicular thyroid carcinoma. Cureus. 2016;8(11):e898.
Löhr M, Tzouras G, Kocher M, Stenzel W, Reithmeier T, Klug N, Hampl JA. Treatment strategies of space-occupying intradural metastases of the cauda equina of nonneurogenic origin. Acta Neurochir. 2009;151:207.
Marotta N, Mancarella C, Colistra D, Landi A, Dugoni DE, Delfini R. First description of cervical intradural thymoma metastasis. World J Clin Cases. 2015;3:946.
Petterwood J, Lim K, Gonzalvo A, Quan G. Intradural extramedullary colorectal adenocarcinoma metastasis to the cervical spine. ANZ J Surg. 2015;85:582–3.
Strong C, Yanamadala V, Khanna A, Walcott BP, Nahed BV, Borges LF, Coumans JV. Surgical treatment options and management strategies of metastatic renal cell carcinoma to the lumbar spinal nerve roots. J Clin Neurosci. 2013;20:1546–9.
Amparo W, Johnstone R, Siddiqi F. Intradural extramedullary spinal cord metastasis of the prostate: a case presentation and review of the literature. Can J Neurol Sci. 2016;43.4:588–92.
Akhavan A, Mehrabaniyan MR, Jarahi M, Navabii H. Intradural extramedullary metastasis from papillary carcinoma of thyroid. BMJ Case Rep. 2012;2012:bcr0220125801.
Greene Stephanie, et al. Pediatric intradural extramedullary synovial sarcoma: case report. Neurosurgery. 2006;59:E1339–E1339.
Paterakis K, Brotis A, Tasiou A, Kotoula V, Kapsalaki E, Vlychou M. Intradural extramedullary Ewing’s sarcoma: a case report and review of the literature. Neurol i Neurochir Pol. 2017;51:106–10.
Jost G, Zimmerer S, Frank S, Cordier D, Merlo A. Intradural spinal metastasis of renal cell cancer. Report of a case and review of 26 published cases. Acta Neurochir. 2009;151:815–21.
Acknowledgements
We thank the patient and the patient’s family for providing informed consent for inclusion in this case report, as well as Dr. Mara Banks of the Medical College of Georgia Department of Pathology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its latera amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Land, C.F., Bowden, B.D., Morpeth, B.G. et al. Intradural extramedullary metastasis: a review of literature and case report. Spinal Cord Ser Cases 5, 41 (2019). https://doi.org/10.1038/s41394-019-0181-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-019-0181-0
This article is cited by
-
Surgical treatment of solitary intradural extramedullary spinal cord metastases from solid cancers of non-neurogenic origin. A multicenter study
Journal of Neuro-Oncology (2021)
-
Nitrogen and Phosphorus Gradients from a Working Farm through Wetlands to Streams in the Georgia Piedmont, USA
Wetlands (2020)