Abstract
Study design
Retrospective matched case-control study including patients with spinal cord injury who presented with an anus-near pressure injury. Two groups were formed based on the presence of a diverting stoma.
Objectives
To evaluate the primary microbial colonisation and secondary infection of anus-near pressure injuries depending on the presence of a pre-existing diverting stoma and to investigate the effect on the wound healing.
Setting
University hospital with a spinal cord injury unit.
Methods
A total of 120 patients who had undergone surgery of an anus-near decubitus stage 3 or 4 were included in a matched-pair cohort study. Matching was realised according to age, gender, body mass index and general condition.
Results
The most common species in both groups was Staphylococcus spp.(45.0%). The only significantly different primary colonisation affected Escherichia coli, that was found in the stoma patients less often (18.3 and 43.3%, p < 0.01). A secondary microbial colonisation occurred in 15.8% and was equally distributed, except for Enterococcus spp. that was present in the stoma group only (6.7%, p < 0.05). The time to complete cure took longer in the stoma group (78.5 versus 57.0 days, p < 0.05) and was associated with a larger ulcer size (25 versus 16 cm2, p < 0.01). After correction for the ulcers’ size, there was no association to outcome parameters such as overall success, healing time or adverse events.
Conclusions
The presence of a diverting stoma alters the microbial flora of an anus-near decubitus slightly without impact on the healing process.
Similar content being viewed by others
Introducton
Despite preventative efforts and well-established clinical guidelines [1,2,3], patients with spinal cord injury (SCI) develop pressure injuries quite frequently [4]. Over 30% of SCI patients are affected during their initial hospitalisation [5]; the prevalence in the chronic stage of SCI varies between 15 and 60% [6, 7]. Pressure injuries, also referred to as decubitus, are one of the most common reasons for rehospitalisation [7, 8]. The subjects affected suffer from a decreased health-related quality of life and an increased risk of severe complications and mortality [8,9,10].
Infectious problems occur frequently and might deteriorate the course of the disease. Necrotising fasciitis, for instance, develops in up to 5% of cases and leads to sepsis and multiorgan failure in every fourth patient affected [11]. The ulcer’s progression and, accordingly, the extent and the intensity of the treatment depend on the bacterial burden [12], the colonisation with certain species, such as anaerobes [13], and the presence of multiresistant germs [14] that are associated with a longer and more severe course of the disease and with a more complex and expensive treatment [15]. Half of the pressure injuries are located near the anus [9], therefore, the impaired defecation control in SCI patients [16] might facilitate ongoing contamination and secondary infection. Faecal diversion via a stoma is a common concept that is thought to improve the healing process by preventing the wound from becoming contaminated with faecal microbiota [17].
Nevertheless, it is unclear whether and how the colonisation or secondary infection pattern changes in the presence of a stoma and if these presumed alterations play a pivotal role in the healing process. Although there are some hints in bed-bound patients [17], our group recently could not confirm an improved outcome in stoma-treated SCI patients [18] and, even more remarkably, a high mortality of 15% was described after colostomy in a small cohort [19]. Hence, we aimed to investigate the microbial pattern regarding the primary (preoperative) colonisation, the secondary (postoperative) infection, the frequency of multiresistant microbiota, and the effect on ulcer healing in a retrospective, age-, gender- and general condition-matched case-control study.
Materials and methods
Study population
A total of 60 patients with colostomy or ileostomy was included in 464 consecutive adult patients with longstanding (≥6 months) SCI with an anus-near decubitus stage 3 or 4, according to the European Pressure Ulcer Advisory Panel (EPUAP) and the National Pressure Ulcer Advisory Panel (NPUAP) [1] (Table S-1) requiring surgery. An age- (±3 years), gender-, body mass index (BMI)- (±4 kg/m2) and ASA score-matched group of SCI patients without a stoma served as a control in this retrospective cohort study in a 1:1 ratio (Fig. S-1 and Table S-2). The inclusion and exclusion criteria are detailed in Table S-3. All patients were treated in a specialised SCI unit (University Hospital Bergmannsheil, Bochum, Germany) between 2007 and 2017.
Clinical data acquisition
Clinical data, such as BMI, smoking habits, alcohol consumption, comorbidities, previous ulcer surgery and medication, were collected from the electronic database at admission. Laboratory parameters were studied before the pressure injury’s surgery and at discharge.
Definition of ulcer characteristics and staging of disability
The anus-near localisation was defined as any ulcer that spread out with at least a substantial surface within the following anatomical structures: the connecting line between both posterior superior iliacal spines (e.g. the cranial margin), the connecting lines between the two ischial tuberosities and the dorsal perineal area (e.g. the ventrocaudal margin), and the connecting line between the posterior superior iliacal spines and the ischial tuberosities (e.g. the lateral margins). The severity level of the ulcers was classified according to the EPUAP, NPUAP and PPPIA (Table S-1) [1]. The healing was evaluated based on the clinical charts. If the detailed description of the achievement of the complete wound healing time was not available, the time of discharge was defined as the end point of wound healing as implemented in routine practice. Preterm discharge before complete wound healing was documented accordingly.
Microbial characterisation
Smears were taken from the deep wound ground during initial surgery and repeated on demand, for instance, in the case of delayed wound healing or signs of secondary infection. Microbial germs that are not part of the local skin microbiota or amounts over the upper limit values are classified as primary colonisation. Microbial colonisation that has not been described in the initial probe was classified as a secondary infection. Ulcers in which only one germ species was found were rated as a monoculture. Bacteria were distinguished as Gram-positive and -negative and were assigned to their superordinated species (e.g. Staphylococcus spp., Enterococcus spp.). Anaerobic bacteria and fungi were characterised separately.
Microbial testing routine
All microbial tests were performed at the microbial laboratory of the University Hospital Bergmannsheil Bochum with reagents and technical devices from bioMérieux, Nürtingen, Germany. Inoculated chocolate agar and MacConkey agar were incubated at 37 °C for 48 h to identify the germ species of blood agar plates. Growth media for anaerobic bacteria and fungi was incubated at the same temperature for five days. Enrichment broths were used. Growth media for multiresistant Staphylococcus aureus and enterococcus were used routinely. Identification of the cultured germs was realised by the microbial identification and antibiotic susceptibility testing system VITEK (bioMérieux, Nürtingen, Germany).
Surgical standard procedure
Surgical procedures, including the mechanical wound debridement with removal of coatings and necrotic tissue, were realised under general anaesthesia, as described previously [18].
Objectives
The primary objective was to evaluate the microbial colonisation of an anus-near pressure injury stage 3 and 4 depending on the presence of a stoma. Secondary objectives were the detection of multi-resistant bacteria, the frequency and the character of a secondary infection, and the impact on wound healing (days of hospital and intensive care stay, condition at discharge) on complications and mortality. General risk factors (e.g. active smoking, alcohol consumption) as well as SCI- and ulcer-related factors (e.g. length, size, severity) were analysed regarding possible effects on primary and secondary objectives.
Statistics
Statistical analysis was performed using SPSS for Windows, version 25.0 (SPSS Inc, USA). The arithmetic mean and standard deviation or median and interquartile range (IQR) were used for metric variables. Categorial and nominal data were indicated as absolute and relative frequencies. Interference statistics were realised using paired t-test, Fisher’s exact test, Mann-Whitney U test, chi-squared (χ2) test and analysis of variance (ANOVA). A p-value < 0.05 was regarded as significant. Regression analysis (multinominal, binary logistic, linear regression) was performed on potentially biasing values that were tested with Cramer’s V, Eta coefficient and/or Spearman and Pearson. Thus, correction concerning the pressure injury’s size, white blood cell and haemoglobin value, presence of a stoma, and all germ species tested as an effect on the duration of healing was calculated. Additional risk factors on the endpoints, such as alcohol and nicotine consumption, were calculated. We tested the associations of different starting points (size of the pressure injury, previous operations and, above all, the presence of a stoma) with the colonisation of different bacterial species and analysed the association with clinical outcome parameters (duration of hospital stay, complications, recurrence). Missing data were mentioned explicitly.
Results
A total of 60 stoma-treated patients with faecal diversion (FDG) and 60 matched controls (CG) (median age 52 years (IQR 43; 65), 26 females (21.7%), median BMI 26.0 kg/m2 (IQR 22.3; 29.3), ASA classification: 21 (35%) grade 2, 39 (65%) grade 3) were included (Table S-2). Basic demographic and SCI characteristics were distributed similarly (Table 1 and S-4). Comorbidities (Table S-5), medication (Table S-6) and laboratory parameters (Table S-7) differed only slightly: for instance, the FDG presented lower haemoglobin values at admission (10.9 vs. 12.1 g/dl, p < 0.01) and tendentially at discharge (11.3 vs. 12.1 g/dl, p = 0.05).
Ulcer characteristics
Approximately half of the patients in both groups had undergone previous ulcer-related surgery. Although the severity was equally distributed between both groups (grade 3 in 51.7 vs. 55.0% and grade 4 in 48.3 vs. 45.0%, respectively) the extent was slightly larger in the FDG (25.0 (6.0; 80.0) vs. 16.0 (4.5; 35.0) cm2, p < 0.01, Fig. S-2). The current ulcer’s size was not specified in 21 cases (FDG 51 vs. CG 48).
Stoma characteristics
The stomata were constructed as end-colostomy in 24 and as loop-ileostomy in 14 cases (Table 1 and S-2). The remaining cases were not specified. The median time between stoma construction and current ulcer treatment amounted to 40 months (IQR 2.9; 178.8).
Microbial colonisation and secondary infections
Virtually all patients (97.5%) presented a primary microbial colonisation (Tables 2 and 3). Gram-positive germs were most common in both groups (FDG = 43 (71.7%) and CG = 38 (63.3%), p = 0.33), and Gram-negative colonisation was detected in 40 (66.7%) and 43 (71.1%) patients, respectively (p = 0.55). The abundance of bacterial species did not differ: monocultures were documented in 24 (FDG, 40.0%) vs. 23 (CG, 38.3%), and polymicrobial colonisation with more than three species were detected in 4 (6.7%) and 3 patients (5.0%), respectively.
Almost half of the wounds were colonised with Staphylococcus spp. (FDG = 26 (43.3%) vs. CG = 28 (46.7 %), p = 0.71). Streptococcus spp. were less frequent with 12 (FDG, 20%) and 8 (CG, 13.3%, p = 0.36) verified germs (Fig. 1). A remarkable difference was detected with respect to E. coli spp.: 11 (18.3%) cases were found in the FDG compared to 26 in the CG (43.3 %; p < 0.01). Colonisation with Klebsiella spp. differed tendentially with 7 (FDG 11.7%) vs. 2 (CG 3.3%) cases (p = 0.08). The colonisation with Pseudomonas spp., Proteus spp. and Enterococcus spp. was not distinguished (Table 3). Anaerobes were equally detected in both groups (FDG = 6, 10.0% and CG = 7, 11.7%). The presence of multiresistant was twice as high in the CG (FDG = 7 (11.7%) vs. CG = 14 (23.3%), p = 0.09). A detailed description is given in Figs. 1, 2, S-4 and S-5.
Diversity and variable proportions of bacterial species regarding the primary colonisation in the faecal diversion (FDG) and control group (CG) shown as a bar chart. A significant difference can be seen in E. coli spp. colonisation with 11 (18.3%) in the FDG vs. 26 (43.3%) in the CG. (p < 0.01). Statistics were realised by the Chi2-test (**: significant with p < 0.01).
Diversity and variable proportions of bacterial species regarding the secondary infection in the FDG and CG shown as a bar chart. A significant difference is seen in Enterococcus spp. colonisation. No case was detected in the CG, but 4 (6.7%) in the FDG (p < 0.01). Statistics were realised by Chi2-test (*: significant with p < 0.05).
Nineteen patients experienced a secondary infection during ulcer treatment, equally distributed among both groups (FDG = 11 (18.3%) vs. CG = 8 (13.3%); Table 4). Gram-positive and -negative strains generally occurred similarly, but, in detail, there was a slight difference regarding Enterococcus spp. (FDG = 4 (6.7%), CG = 0; p < 0.05).
Length of hospital stay, adverse events and recurrence
Stoma-treated patients stayed 21 days longer in the hospital than the CG (78.0 (IQR 49.3; 113.0) vs. 56.5 (IQR 43.3; 90.5) days, p < 0.05). The vast majority were discharged with a cured ulcer. The remaining 9 patients aborted the treatment upon request and followed outpatient treatment (FDG = 2 (3.3%)) or died (FDG = 4 (6.7%) vs. CG = 3 (5%)). Hence, the mortality rate did not differ. Further adverse events are detailed in Table S-8, grouped by severity (Fig. S-3)
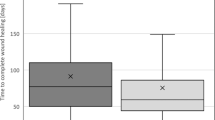
Taking only the patients with cured ulcers into account, the length of hospital stay needed a minor correction (FDG 78.5 vs. CG 57.0 days, p < 0.05; Table 2 and Fig. 3). The correlation between the presence of a stoma and the length of healing time is weak, but significant (Eta coefficient = 0.229, p = 0.01). Additionally, the healing time negatively correlated weakly with the decubitus’ size (p = 0.01, Pearson = 0.252) and various other preoperative laboratory values (red and white blood cell count, platelets, and CRP; Table S-9). Partial correlation with adjustment for the parameters previously mentioned suggests that the presence of a stoma does not affect the ulcer’s healing time (p = 0.14).
Duration of ulcer healing regarding the presence of a stoma: the length of healing (in days) of the FDG and the CG are shown as box plots, so that a comparison of the two groups is possible. These box plots include only patients who completed the treatment regularly and hence are labelled as successfully cured. Patients who died prematurely (FDG = 4 (6.7%) vs. CG = 3 (5%)) or discontinued treatment for other reasons (FDG = 2, 3.3%) are not reflected. The median duration varied significantly (*) from 75.5 days (IQR 50.0; 111.5) in the FDG to 57.0 days (IQR 45.0; 93.5) in the matched CG with supported natural defecation (Mann–Whitney-U-test, p < 0.05).
Risk factor analysis
The binary logistic regression revealed a significant effect for the grade of the decubitus to predict the colonisation with multiresistant germs in general (OR = 2.7, CI = 1.1–6.6, p = 0.03). Smoking was determined as a relevant risk factor for secondary infections in the cohort of stoma-treated patients (OR = 8.7, CI 1.5–49.8, p = 0.01); these findings were not reproducible among the CG, which included only ten active smokers.
The binary logistic regression analysis considering the whole cohort showed that the presence of a stoma (OR = 2.9, CI 1.5–5.9, p = 0.003), monocultures (OR = 2.7, CI 1.1–6.6, p = 0.02) and multiresistant germs as primary colonisation (OR = 4.7, CI 1.0–21.3, p = 0.05) are associated with a reduced probability of E. coli spp. The other germ lines were associated with other parameters (Table S-10): the frequency of Streptococcus spp. decreased significantly with previous surgery (OR = 9.8, CI 4.2–22.7, p < 0.001) and colonisation with Staphylococcus spp. was identified to be associated with a decreased number of secondary infections (OR = 12.5, CI 4.3–34.6, p < 0.001). Wound-healing disturbances were not influenced by particular germ species. The verification of multiresistant germ lines was negatively associated with E. coli (OR = 17.5, CI 4.2–72.8, p < 0.001) and positively with Staphylococcus spp. (OR = 2.0, CI 1.1–3.5, p = 0.01). None of the species of the primary colonisation or secondary infection was associated with wound-healing disturbances or the length of the hospital stay.
Discussion
The main result of this thoroughly matched retrospective cohort study of patients with SCI is that the presence of a stoma is associated with only minor changes of the microbial colonisation of anus-near pressure injuries. Though the main idea of the faecal diversion concept is to achieve a cleaner wound ground, the frequency of microbial colonisation, on the one hand, and of the variety of species isolated from the wound grounds, on the other hand, did not differ between the patients with faecal diversion and those relying on a supported natural defecation. Furthermore, as has been described previously, the main species [20] found in nearly every second ulcer was Staph. aureus. This finding was consistent in both groups similarly and underlines the importance of factors other than faecal contamination even in anus-related ulcers.
Focusing on typical faecal bacteria, we only found one remarkable difference: as expected, E. coli colonised the wounds of patients with natural defecation approximately twice as often (43.3 vs. 18.3 %; p < 0.01). On the other hand, when E. coli was absent, multiresistant germs occurred five times more often. Since multiresistant bacteria were more prevalent among the control group it might be reasonable that more frequent antibiotic use in these patients and the resulting selection pressure on the colonising bacteria may have lead to these microbial changes [21]. Since we do not possess any such information, it might also be arguable that E. coli prevents the wound’s colonisation competitively and by activation of the mucosal immune system [22].
A similar amount of 18.3 and 13.3% acquired a secondary infection during the ulcer’s treatment and healing process; the only remarkable difference involves Enterococcus spp., which occurred more frequently in the faecal diversion group. A possible explanation might refer to the higher bacterial load in the stoma-treated patient’s environment that might result from spreading faeces [23]. Hence, the bacteria might be transferred during stoma and wound care.
Some bacterial species, particularly Staph. aureus, Proteus spp. and Bacteroides spp., play a vital role because those infections increase the risk of complications and mortality in pressure injuries [24]. Our data suggest that the presence of a stoma does not prevent microbial colonisation or secondary infection and affects the microbial wound flora only slightly. More importantly, neither the faecal diversion nor the microbial spectrum was associated with the wound healing.
Despite diligent matching according to ASA classification, gender, age and BMI, there are some limitations to discuss. First of all, the patients with faecal diversion presented slightly larger ulcers and, consequently, probably needed longer for the healing process. Nevertheless, there were no associations to the bacterial colonisation or secondary infection. Secondly, though the reason for the previous stoma construction remained unclear, it was rarely constructed for the purpose of the current ulcer treatment and contained different types, such as loop ileostomy and end colostomy. It might be arguable that the time shift between stoma construction and ulcer treatment might have influenced the primary colonisation pattern, but it is not reasonable to affect the secondary colonisation or wound healing. The lack of any association on the outcome parameters confirms these fundamental considerations regarding the faecal diversion concept. Thirdly, the retrospective design resulted in a loss of certain information. The ASA classification, for instance, was only available at the time of inclusion, but not the stoma construction. Additionally, the indications for stoma construction remained uncertain; it might reasonably be possible that the severity of comorbidities, a lower overall physical performance or other clinical aspects could have influenced the decision in favour of the stoma. The matching process has adjusted these biasing factors and resulted in mostly similar basic demographic characteristics; only a few differences occurred including a longer duration of SCI, more frequent smoking habits, divergent laboratory values and larger ulcer sizes. According to the regression analysis, these parameters interfered with the prolonged healing time in the faecal diverted patients, but did not change the bacterial flora. Additionally, the healing time that complies with the current literature [7] is not influenced by the presence of a stoma. Fifthly, the microbial identification was realised by culture techniques that might not detect all the different species within the complexity of a chronic wound [20]. Though the flora found in this cohort matches well to that described in literature [20], newer techniques, such as RNA-based fingerprints, might have detected a larger variety of germs [25].
Conclusion
Although the presence of a stoma is associated with less frequent colonisation of anus-near pressure injuries with E. coli, the overall microbial pattern shows only minor alterations. Future, preferably prospective studies with larger cohorts should validate the results and broaden the analysis by new laboratory methods. After correction for the ulcer’s size, the stool deviation concept did not influence the treatment outcome parameters such as overall success, healing time or adverse events. Regarding the potential morbidity and psychological restrictions related to the stoma construction, we currently do not recommend it to be the standard strategy and it should, instead, be based on individual decision-making. A randomised controlled study, though hardly realisable, is mandatory to clarify the impact of the faecal diverting concept in the context of the treatment of anus-near pressure injuries.
Data availability
Data for this study are available on reasonable request. Please contact the corresponding author for the research data.
References
European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel PPPIA. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Cambridge Media; 2014.
Pancorbo-Hidalgo PL, Garcia-Fernandez FP, Lopez-Medina IM, Alvarez-Nieto C. Risk assessment scales for pressure ulcer prevention: a systematic review. J Adv Nurs. 2006;54:94–110. https://doi.org/10.1111/j.1365-2648.2006.03794.x
Bhattacharya S, Mishra R. Pressure ulcers: current understanding and newer modalities of treatment. Indian J Plast Surg. 2015;48:4–16. https://doi.org/10.4103/0970-0358.155260
Hauss A, Greshake S, Skiba T, Schmidt K, Rohe J, Jürgensen JS. Systematic pressureulcer risk management. Results of implementing multiple interventions at CharitéUniversitätsmedizin Berlin]. Z Evid Fortbild Qual Gesundhwes. 2016;113:19–26.
Harkey HL, White EA IV, Tibbs RE, Haines DE. A clinician’s view of spinal cord injury. Anat Rec B N. Anat. 2003;271:41–48. https://doi.org/10.1002/ar.b.10012
Gélis A, Dupeyron A, Legros P, Benam C, Pelissier J, Fattal C. Pressure ulcer risk factors in persons with spinal cord injury Part 2: the chronic stage. Spinal Cord. 2009;47:651–61. https://doi.org/10.1038/sc.2009.32
Biglari B, Büchler A, Reitzel T, Swing T, Gerner HJ, Ferbert T, et al. A retrospective study on flap complications after pressure ulcer surgery in spinal cord-injured patients. Spinal Cord. 2014;52:80–3. https://doi.org/10.1038/sc.2013.130
Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757–63. https://doi.org/10.1016/j.apmr.2004.03.016
Sezer N. Chronic complications of spinal cord injury. World J Orthop. 2015;6:24. https://doi.org/10.5312/wjo.v6.i1.24
Krause JS, Devivo MJ, Jackson AB. Health status, community integration, and economic risk factors for mortality after spinal cord injury. Arch Phys Med Rehabil. 2004;85:1764–73. https://doi.org/10.1016/j.apmr.2004.06.062
Citak M, Backhaus M, Tilkorn DJ, O’loughlin PF, Meindl R, Muhr G, et al. Necrotizing fasciitis in patients with spinal cord injury: an analysis of 25 patients. Spine (Philos Pa 1976). 2011;36:E1225–29. https://doi.org/10.1097/BRS.0b013e3182059950.
Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17:2085. https://doi.org/10.3390/ijms17122085
Montgomerie JZ. Infections in patients with spinal cord injuries. Clin Infect Dis. 1997;25:1285–90. https://doi.org/10.1086/516144
Oli AN, Eze DE, Gugu TH, Ezeobi I, Maduagwu UN, Ihekwereme CP. Multi-antibiotic resistant extended-spectrum beta-lactamase producing bacteria pose a challenge to the effective treatment of wound and skin infections. Pan Afr Med J. 2017;27:66. https://doi.org/10.11604/pamj.2017.27.66.10226
Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era. Arch Med Res. 2005;36:697–705. https://doi.org/10.1016/j.arcmed.2005.06.009
Branagan G, Tromans A, Finnis D. Effect of stoma formation on bowel care and quality of life in patients with spinal cord injury. Spinal Cord. 2003;41:680–3. https://doi.org/10.1038/sj.sc.3101529
De La Fuente SG, Levin LS, Reynolds JD, Olivares C, Pappas TN, Ludwig KA, et al. Elective stoma construction improves outcomes in medically intractable pressure ulcers. Dis Colon Rectum. 2003;46:1525–30. https://doi.org/10.1007/s10350-004-6808-6
Pussin AM, Lichtenthäler LC, Aach M, Schildhauer TA, Brechmann T. Fecal diversion does not support healing of anus-near pressure ulcers in patients with spinal cord injury—results of a retrospective cohort study. Spinal Cord. 2021. https://doi.org/10.1038/s41393-021-00717-2
Deshmukh GR, Barkel DC, Darcey S, Penny H. Use or misuse of colostomy to heal pressure ulcers. Dis. Colon Rectum. 1996;39:737–8. https://doi.org/10.1007/BF02054436
Dana AN, Bauman WA. Bacteriology of pressure ulcers in individuals with spinal cord injury: what we know and what we should know. J. Spinal Cord Med. 2015;38:147–60. https://doi.org/10.1179/2045772314Y.0000000234
Levy SB, Bonnie M. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004;10:S122–9. https://doi.org/10.1038/nm1145
Sansonetti PJ. E08 Flora pathogenicity and paneth cells. J. Crohn’s Colitis Suppl. 2010;4:7–8. https://doi.org/10.1016/s1873-9954(10)70012-4
Lyon CC, Smith AJ, Griffiths CEM, Beck MH. The spectrum of skin disorders in abdominal stoma patients. Br J. Dermatol. 2000;143:1258–60. https://doi.org/10.1046/j.1365-2133.2000.03896.x
Espejo E, Andrés M, Borrallo RM, Padilla E, Garcia-Restoy E, Bella F. Bacteremia associated with pressure ulcers: a prospective cohort study. Eur J Clin Microbiol Infect Dis. 2018;37:969–75. https://doi.org/10.1007/s10096-018-3216-8
Höfle MG. Transfer RNAs as genotypic fingerprints of eubacteria. Arch Microbiol. 1990;153:299–304. https://doi.org/10.1007/BF00249086
Author information
Authors and Affiliations
Contributions
LCL: Conducting the study, acquisition of data, analysis and interpretation, drafting the manuscript; LCL approved the final manuscript. luisa.lichtenthaeler@t-online.de. AMP: Conducting the study, acquisition of data, analysis and interpretation, revision of the manuscript; Andreas Pussin approved the final manuscript. andreaspussin@gmx.de. TAS: Supervision, revision of the manuscript, support in terms of spinal cord injury and surgery; Thomas Schildhauer approved the final manuscript. thomas.A.Schildhauer@ruhr-uni-bochum.de. MA: Conception and design of the study, supervision, revision of the manuscript, support in terms of spinal cord injury; Mirko Aach approved the final manuscript. mirko.aach@bergmannsheil.de. DG: Data analysis and interpretation, drafting the manuscript; Dennis Grasmücke approved the final manuscript. dennis.grasmuecke@bergmannsheil.de. WS: Supervision, revision of the manuscript, support in terms of gastroenterology; Wolff Schmiegel approved the final manuscript. wolff.schmiegel@rub.de. TB: Conception and design of the study, data analysis and interpretation, supervision, drafting the manuscript; TB approved the final manuscript. thorsten.brechmann@rub.de.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the institutional review board of the Ruhr University Bochum [registry number 18-6351] on the basis of the ethical guidelines of the Declaration of Helsinki and its later revisions; informed consent was obtained from all patients before surgery. Informed consent was neither practicable nor necessary for this retrospective study and was exempted by the institutional review board.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lichtenthäler, L.C., Pussin, A.M., Aach, M. et al. Minor microbial alterations after faecal diversion do not affect the healing process of anus-near pressure injuries in patients with spinal cord injury - results of a matched case-control study. Spinal Cord 61, 352–358 (2023). https://doi.org/10.1038/s41393-023-00901-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-023-00901-6