Abstract
Study design
This study is a systematic review.
Objectives
To evaluate current in vivo techniques used in the investigation of the blood–spinal cord barrier (BSCB).
Methods
Search of English language literature for animal studies that investigated the BSCB in vivo. Data extraction included animal model/type, protocol for BSCB evaluation, and study outcomes. Descriptive syntheses are provided.
Results
A total of 40 studies were included, which mainly investigated rodent models of experimental autoimmune encephalomyelitis (EAE) or spinal cord injury (SCI). The main techniques used were magnetic resonance imaging (MRI) and intravital microscopy (IVM). MRI served as a reliable tool to longitudinally track BSCB permeability changes with dynamic contrast enhancement (DCE) using gadolinium, or assess inflammatory infiltrations with targeted alternative contrast agents. IVM provided high-resolution visualization of cellular and molecular interactions across the microvasculature, commonly with either epi-fluorescence or two-photon microscopy. MRI and IVM techniques enabled the evaluation of therapeutic interventions and mechanisms that drive spinal cord dysfunction in EAE and SCI. A small number of studies demonstrated the feasibility of DCE-computed tomography, ultrasound, bioluminescent, and fluorescent optical imaging methods to evaluate the BSCB. Technique-specific limitations and multiple protocols for image acquisition and data analyses are described for all techniques.
Conclusion
There are few in vivo investigations of the BSCB. Additional studies are needed in less commonly studied spinal cord disorders, and to establish standardized protocols for data acquisition and analysis. Further development of techniques and multimodal approaches could overcome current imaging limitations to the spinal cord. These advancements might promote wider adoption of techniques, and can provide greater potential for clinical translation.
Similar content being viewed by others
Introduction
The blood–spinal cord barrier (BSCB) is a term used to describe the protective functions of the spinal cord (SC) microvasculature [1]. While the BSCB has a number of morphological and functional characteristic features that make it distinct from other central nervous system barriers [1, 2], it is relatively understudied [3]. BSCB dysfunction plays a vital role in several pathological disorders, which include amyotrophic lateral sclerosis, multiple sclerosis, neuropathic pain, and injury of the SC [1, 4]. These disorders have a significant impact on the quality of life, morbidity, and mortality of patients [1]. The timing, mechanism of onset, and exacerbation of BSCB dysfunction in the pathophysiology of spinal cord disorders remain elusive.
Nonterminal in vivo imaging techniques provide real-time data, with the ability to investigate the temporal dynamics of BSCB function and neurobehavioral testing in longitudinal studies [5,6,7,8]. In vivo experiments can help identify the sequence of events associated with BSCB dysfunction [2], as opposed to terminal methods that are limited by cross-sectional design, and the deduction of driving mechanisms [5, 9, 10]. It is difficult to assess the SC with in vivo imaging techniques due to the small size of the SC, along with significant motion artifacts that occur due to the nearby cardiovascular and respiratory system [6, 10,11,12]. Reviews can offer unique insight into current technique applications [1, 9, 13], and can be systematically conducted to collate all current data to identify knowledge gaps, assess study quality, and guide future research [14, 15]. Therefore, we performed a systematic review to evaluate all current in vivo techniques used in the investigation of the BSCB.
Methods
A systematic review was performed following the guidelines by the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies [14]. The following databases were searched: PubMed (MEDLINE), EMBASE, and Scopus up to June 15, 2020. Various combinations of the following terms were utilized: “blood–spinal cord barrier” or “blood–brain barrier” or “spinal cord”, while restricted to animal studies, and the English language literature. Original research studies investigating a function of the BSCB in vivo using animal models were included (Fig. 1).
A pre-piloted spreadsheet was used to record the extracted data from the included studies. Data extraction included study characteristic, objective, animal model type, detailed in vivo technique evaluating BSCB and outcome, tissue locations analyzed, concurrent histological or neurobehavioral outcomes, overall conclusions, and information for assessment of study quality.
Included studies were grouped by technique and animal model investigated, and descriptive syntheses of the findings from all studies are provided in text and tables (Tables 1–4 and Supplementary Table 1). Study quality was scored using an adapted checklist [15] and the median with range is provided (Supplementary Table 2). Please see Supplementary text for additional details.
Results
Study characteristics
A total of 40 studies met inclusion criteria (Fig. 1, please see the Supplementary for full reference list). They mainly investigated disease mechanisms in experimental autoimmune encephalomyelitis (EAE) or spinal cord injury (SCI) and utilized rodent models (Table 1) [2, 5,6,7,8, 10,11,12, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. The most common in vivo techniques reported were magnetic resonance imaging (MRI, Table 2) [6, 18,19,20,21,22,23,24, 27, 28, 30, 33, 35, 37] and intravital microscopy (IVM, Table 3) [2, 5, 8, 10,11,12, 16, 17, 25, 31, 32, 34, 38]. Less common techniques such as computed tomography (CT) [29], ultrasound (US) [36], bioluminescent [39], and fluorescent [26] optical imaging have also been described.
Radiological imaging techniques
MRI
The use of Gadolinium-based (Gd) contrast-enhanced MRI was most commonly used to assess BSCB permeability changes [6, 19, 20, 22,23,24, 28, 33, 35, 37]. Permeability (disruption) of the BSCB was determined by extravasation of Gd into the extravascular–extracellular space, and measured by increased signal intensity on T1-weighted images [6, 19, 20, 22,23,24, 28, 33, 35, 37]. MRI studies (Table 2) either assessed post-contrast changes at single time points with CE-MRI [22, 35], or dynamic changes by obtaining a continuous series of images over time after contrast administration with DCE-MRI [6, 19,20,21, 23, 24, 28, 33, 37]. The enhancement kinetics acquired from MRI provided data on both spatial and temporal changes in BSCB permeability [6, 20, 21, 40].
MRI data were analyzed either using model-free methods (i.e., peak signal intensity, area under signal intensity curves, etc.) to produce qualitative measurements [18,19,20, 22, 23, 27, 28, 30, 35], or the application of pharmacokinetic models for quantitative assessments [6, 21, 24, 33, 37]. A two-compartment model [6, 24, 33, 37, 40] was the most common pharmacokinetic model reported, which measured the rates of the contrast agent transport (K-trans) between plasma and the parenchyma, as the two compartments [6, 24, 33, 37]. The use of a three-compartment model with the inclusion of cerebrospinal fluid as the third compartment was explored [21], but did not yield a satisfactory fit to the experimental data.
MRI and SCI
DCE-MRI served as a reliable tool to longitudinally track microvascular permeability changes in the SC after injury [6, 19, 20, 22, 24, 33, 37]. Evaluating the BSCB with DCE-MRI after contusion-type SCI demonstrated significant acute BSCB compromise that decreased over time, and remained compromised up to 42 days after injury [20]. The recovery rate of the BSCB integrity correlated with improved neurobehavioral outcomes [20, 24, 33]. Gd enhancement was also found several millimeters away from the epicenter of the injury along the length of the SC, and thought to be due to immature barrier properties in the neovasculature [20, 24, 33]. The timing in the recovery of BSCB integrity after SCI-induced changes varied along the cranial-caudal axis [24]. BSCB dysfunction first recovered at the epicenter and caudal extent of the SCI; however, BSCB dysfunction remained rostral to the injury up to 56 days. DCE-MRI and diffusion tensor imaging were simultaneously employed and showed that increased BSCB permeability occurred with decreased neuronal architecture in a spatio-temporal pattern after injury [37]. Variations in the pathological responses of rats vs. mice to SCI-induced BSCB dysfunction patterns were also captured with MRI [22]. Not all SCI models demonstrated BSCB dysfunction after injury, as excitotoxic SCI models did not show BSCB changes with DCE-MRI [19]. These findings were confirmed with histopathologic experiments.
The impact on BSCB dysfunction by therapeutic agents that have been shown to promote angiogenesis was investigated [28, 33]. Vascular endothelial growth factor (VEGF) is known to stimulate angiogenesis and vascular permeability; however, its therapeutic role in SCI is controversial [28, 33]. Using DCE-MRI, BSCB permeability was observed to be greater at all time points after VEGF treatment, which was attributed to immature barrier properties in the neovasculature [33]. The VEGF-treated group demonstrated earlier improvements in locomotion compared to control groups [33]. However, changes in BSCB permeability were not associated with motor outcomes. Follow-up studies evaluated the administration of VEGF combined with angiopoietin-1 (vascular stabilization) through viral vectors injected into the epicenter of the SCI, and found a reduction in the lesion volume, improved BSCB integrity and locomotor outcomes in treated groups [28]. These studies suggested that combination therapy promoted stable neovascularization [28, 33].
MRI and peripheral nerve injury (PNI)
The BSCB was evaluated in a PNI model, where the tibial and common peroneal nerves were injured to model features of neuropathic pain [23]. A delayed increase in BSCB permeability was noted along the thoracolumbar SC post injury, and returned to normal in approximately 1 day. Differences in the degree of BSCB permeability changes were observed across different strains with known differences in neuropathic features [23].
MRI and EAE
EAE is a widely used animal model of demyelinating disease of the CNS used to study select features of multiple sclerosis [18, 27, 30, 35]. CE-MRI studies have shown increased BSCB permeability before and during the onset of clinical symptoms, and lack of permeability during remission states [35]. Targeted alternative contrast agents (vs. conventional Gd) for MRI evaluation of EAE disease activity have been described [18, 27, 30]. In EAE models, Gadofluorine-M (Gf-M) demonstrated increased sensitivity in the detection of inflammatory lesions, when compared to conventional Gd [18]. These findings were attributed to higher accumulation of Gf-M (vs. Gd) in regions with barrier dysfunction and inflammation [18].
The autoimmune inflammatory response in EAE includes the infiltration of macrophages, which was assessed with T2-weighted MRI using cellular iron-oxide contrast agents (IOCA) [30]. The activity and timing of macrophage infiltration with BSCB compromise was performed with conventional CE-MRI along with IOCA to assess for macrophage infiltration [30]. There was little overlap observed in regions of BSCB compromise and identified macrophage infiltration, which suggested that not all regions of inflammation were associated with BSCB breakdown [30]. Antibodies targeting endothelial adhesion molecules were coupled with IOCA and were used to monitor activated endothelial cells during neuroinflammation [27]. Relative signals derived by targeting P-selectin with IOCA-antibodies for imaging were predictive of relapse and remission states in EAE. MRI studies have shown the ability to non-invasively track microvascular dysfunction and inflammatory activity in EAE with the use of targeted alternative contrast agents [18, 27, 30].
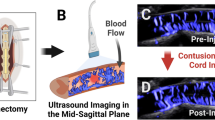
CT or ultrasound and SCI
Temporospatial changes in BSCB permeability were also quantified with DCE-CT, by utilizing iodinated contrast agents and applying similar pharmacokinetic modeling used in DCE-MRI [29]. DCE-CT and concurrent perfusion measurements were used to evaluate the therapeutic effect of local delivery of fibroblast-growth-factor 2 after compression-type SCI [29]. The treated group demonstrated increased blood flow and decreased permeability rostral to the epicenter of the SCI [29]. DCE-CT proved feasible in serially evaluating BSCB changes. The evolving changes after SCI were also investigated with very high-resolution US imaging of the SC through a surgical window [36]. US imaging provided detailed structural and vascular imaging of the entire SC, and semi-quantitative measurements of vascular disruption during the acute and subacute phases of SCI [36].
IVM techniques
IVM was the second most common method used [2, 5, 7, 8, 10,11,12, 16, 17, 25, 31, 32, 34, 38], and provided the ability to visualize immune cell interactions across the BSCB with fluorescence imaging (Table 4). IVM was mainly performed with either real-time epi-fluorescence (EPI-IVM) [34, 38] or time-lapse two-photon laser scanning microscopy (2P-IVM) techniques [2, 5, 8, 10, 17, 25, 31], and required surgical window preparations (Table 3). The development of protocols for obtaining steady-state respirations allowed stable 2P-IVM of the SC [10, 11]. This often required custom-made stabilization devices, deep anesthesia, and tracheotomy with intubation to minimize breathing movements [11, 31]. The development of an implanted SC window-chamber allowed long-term imaging without requiring repeat surgical preparations [12].
The microvasculature was frequently visualized with intravenous injection of large fluorescent markers [2, 5, 7, 8, 10,11,12, 16, 17, 25, 31, 32, 34, 38]. The majority of studies described fluorescent labeling of cells [5, 11, 17, 32, 34, 38] for either tracking their movement pattern, velocity, morphology, or transgression across the BSCB. Permeability was not commonly assessed with small fluorescent markers, given that its extravasation hindered interim imaging analysis [5, 16].
IVM and EAE
Mononuclear phagocytes, including macrophages and local microglia, are a central force that drives the formation of inflammatory lesions in EAE [5]. Using 2P-IVM, the migration of microglial cells toward the spinal microvasculature was found to be promoted through the leakage of plasma fibrinogen (BSCB breakdown) before signs of neurologic deficits [5]. Fibrinogen then induced microgliosis, perivascular clustering, release of reactive oxygen species, and axonal damage [5]. The inhibition of fibrinogen signaling reduced perivascular clusters and axonal damage [5].
The same macrophages can be equally important to lesion recovery, as individual phagocytes have been observed to go from proinflammatory to anti-inflammatory polarization during lesion resolution [31]. Using a novel phenotypic imaging technique, based on reporters that translate the pro- or anti-inflammatory polarization of phagocytes into distinct fluorescent signals [31], the temporospatial evolution of polarization states was visualized with 2P-IVM [31]. By tracking phagocytes at different time points in different compartments, phenotypic changes were captured when crossing the meningeal to parenchymal compartment [31], and the highest polarization rates from pro- to anti-inflammatory states were observed at the pia–parenchymal border and below [31]. This phenotypic shift to anti-inflammatory states suggested that these phagocytes may be involved in disease remission [31].
The multistep mechanisms resulting in T-cell rolling, capture, adhesion, and diapedesis in EAE were investigated with EPI-IVM by tracking the motility of fluorescent-tagged T cells [34, 38]. T-cell tethering and rolling are mediated by an expressed adhesion molecule (PSGL-1) interacting with its endothelial ligand (P-selectin) [34], but this was not required for T-cell entry into the parenchyma and initiation of EAE [34]. Tracking fluorescent T cells with IVM demonstrated that they required α4-integrin and lymphocyte function-associated antigen-1 mediated interactions with endothelial cells for T-cell migration into the parenchyma [38].
Myelin-reactive T-cell blasts were presumed to cross the microvasculature and arrest in the perivascular space, until they encountered stimulation from perivascular phagocytes [17]. This resulted in proinflammatory mediators, while making it possible for parenchymal invasion and causing severe disability approximately 3–4 days after adoptive induction of EAE [17]. However, when tracking these T cells systemically, it was found that they acquired the migratory capacity (Tmigratory cells) for parenchymal invasion after first residing in the lung tissues [32]. Lung-derived Tmigratory cells were independently capable of invading the parenchyma of another naive animal and cause paralytic disease within 2 days. T cells can cross the microvascular barrier through disrupted tight junctions or caveolae to induce tissue inflammation [2]. By using caveolae-deficient mice with fluorescently labeled endothelial tight junctions [2], it was found that tight-junction remodeling preceded the onset of EAE and that specific subsets of T cells used distinct mechanisms to cross the BSCB [2]. Specifically, tight-junction remodeling facilitated Th17 paracellular migration vs. Th1 trans-cellular migration via endocytic vesicles containing caveolae [2]. These studies highlighted potential therapeutic targets that could limit T-cell infiltration during disease activity [2].
IVM and SCI
2P-IVM was used to investigate the influence of neovascularization on axon regrowth after SCI [25]. Using transgenic mice with fluorescent axons and simultaneous visualization of microvascular changes, angiogenesis was observed within 2 weeks after SCI [25]. Early sprouting of injured axons with extensive regrowth past the injured site occurred within 2 months [25]. These findings suggested that stimulating angiogenesis could be beneficial to repair and recovery [25].
SCI induces a robust early inflammatory response [7], and during this response fluorescent-tagged neutrophil rolling and adhering to endothelial cells was visualized with EPI-IVM [7]. The depletion of these neutrophils correlated with worse clinical outcomes, suggesting these neutrophils promote recovery after SCI through protective events that limit lesion propagation [7]. The impact of immediate methylprednisolone administration on axonal damage, blood flow, and calcium influx after SCI was evaluated with 2P-IVM [8]. Methylprednisolone treatment attenuated progressive damage of axons, increased blood flow, reduced neuronal calcium influx post injury, and improved neurobehavioral outcomes [8].
Optical imaging
Other noninvasive optical imaging methods have been described (Supplementary Table 1) [26, 39]. Bioluminescence imaging (BLI) can be used to measure the expression of P-glycoprotein, a transporter specific to CNS-barrier endothelial cells [39]. This was accomplished by developing a transgenic mouse model with a human P-glycoprotein promoter also driving a luciferase reporter [39]. Expression of P-glycoprotein was then monitored with BLI following D-luciferin administration [39]. Changes in barrier permeability can be captured with fluorescence molecular tomography (FMT) using near-infrared fluorescence (NIRF) agents, and has been shown to correlate with disease activity in relapsing-remitting EAE [26].
Study quality
The median quality score of all studies was 4 (range 1–6) (Supplementary Table 2). Scores trended higher on more recent publications, due to increased reporting on compliance with regulatory requirements and potential conflicts of interest [15]. Most studies lacked protocols for blind assessment of their outcomes [15]. IVM studies obtained lower scores due to the use of anesthetics with neuroprotective properties [15].
Discussion
In vivo imaging has improved our understanding in the temporal complexity of the BSCB function and its relationships with the neuronal, glial, and immune systems. These techniques can identify and evaluate potential therapeutic interventions, giving more credence to their translational opportunities. However, there are a limited number of studies investigating the BSCB with in vivo techniques, which may be due to the complexity and limitations of current approaches. The current systematic review demonstrated a number of available techniques, along with their strengths, shortcomings, and contributions to our knowledge of BSCB functions (Tables 2–4). This information may guide future experimental studies in SC microvasculature. Technique-specific obstacles for comparative purposes, and potential translational applications will now be discussed. Detailed description of the techniques is beyond the goal of this paper; thus, we will direct readers to other citations for further reading.
MRI
A wide range of MRI acquisition protocols, analysis techniques, and permeability metrics were described, making it difficult to directly compare the results reported by MRI investigations included in the current review [6, 19, 20, 22,23,24, 28, 33, 35, 37]. Based on the goals of included studies, the appropriate temporal and spatial resolution, and signal-to-noise ratio (SNR) were optimized before data analysis. T1-weighted images using spin-echo sequences were most commonly utilized, and required a relatively long acquisition time (minutes). Faster imaging sequences (Rapid Acquisition with Relaxation Enhancement) can provide better temporal resolution [28], but should be based on the expected permeability changes (Ktrans). For example, included MRI studies on contusive-SCI models implemented faster acquisition times to capture acute permeability changes [20, 21], whereas studies investigating EAE or PNI had longer imaging times to capture delayed permeability changes [23, 35]. A number of studies utilized higher magnet field strengths and/or the implantation of surface coils to acquire greater SNR. As a result of these modifications, MRI protocols have been successfully implemented in mice [37] and rats [20] with ~100 μm resolution. Concurrent acquisition of high-resolution anatomical images can be added to delineate gray and white matter, but at the cost of increasing imaging time [37]. Poor temporal resolution can limit study findings, when sequential imaging is required to assess BSCB changes prior to neurologic signs of active disease [35].
The method in measuring BSCB permeability changes from MRI data varied across identified investigations. Early MRI studies assumed linear relationships between signal enhancement and contrast uptake, and used model-free methods of data analysis. Thereby, directly comparing signal enhancement with BSCB disruption. However, contrast concentration is dependent on intrinsic tissue and acquisition parameters, limiting model-free analyses to producing qualitative or semi-quantitative measurements. Contrast agents indirectly increase signal intensity in T1-weighted images (measured by relaxivity). Quantitative analysis of the signal data relies on calibrating the concentration of contrast agents with measured MRI parameters [21, 40], otherwise this may lead to erroneous estimates. A Gd dose should be chosen to maintain the relationship between the signal enhancement and the concentration of contrast agent in a linear model to improve the reliability of quantitative results [20, 40]. This also requires a consistent method of delivery (i.e., intravenous timed bolus via pump). By implementing a mathematical pharmacokinetic model, the measured signal enhancement was more accurately related to the concentration of the contrast agent, and produced quantitative measurements of permeability. The two-compartment pharmacokinetic model is based on the assumption that transport across the BSCB is limited by permeability (not flow), and the rate of contrast agent transport (Ktrans) from plasma to parenchyma is equal to the permeability.
Different methods of MRI acquisition and data analysis can result in conflicting observations between studies [22, 24], and are not directly comparable. Histological assessments have been used to validate in vivo findings, especially if they were questionable. Applying a uniform method for image acquisition and data analysis will promote reproducible data on BSCB permeability that could be aggregated. Further development of more objective measurements would also allow blind assessment of the results. Additional detail on the original pharmacokinetic model [40] applied to calculating BSCB permeability can be found in the referenced studies [6, 21, 37].
CT and US
DCE-CT can also be used for dynamic measurements in the integrity of the BSCB, and similar to DCE-MRI, images are obtained before, during, and after injection of a contrast agent. High-resolution CT has the potential to achieve better temporal and spatial resolution than MRI, but has not yet been shown in BSCB investigations [29]. In contrast to MRI, the acquisition and processing can be more straightforward, due to a more linear relationship between the iodinated contrast agent concentration and X-ray attenuation. The included DCE-CT study utilized a two-compartment pharmacokinetic model for data analysis, which allowed direct comparisons with prior DCE investigations. Further details on the specific assumptions made in the application of pharmacokinetic modeling for DCE-CT can be found in the original reference [29]. Main limitations of DCE-CT included motion artifacts that prevented analysis caudal to T2 (due to lungs), and poor resolution that was insufficient to differentiate gray and white matter measurements.
US devices using high frequencies have been shown to provide high-resolution images of the SC in rodents [36]. Following SCI, quantification of an evolving hyperechoic lesion correlated with BSCB disruption. Major strengths of US techniques include its ability to image the entire SC, and capability to be easily implemented with multimodal imaging approaches [12]. The major limitations of US techniques include operator dependency in data production, and the requirement of a surgical window for imaging access. Additional studies are needed to further evaluate and develop DCE-CT and US techniques.
IVM
Protocols for IVM techniques in the SC were more complex, but provided visualization of the regulatory mechanisms and cellular interactions underlying endothelial barrier integrity. IVM was mainly performed with EPI-IVM or 2P-IVM. EPI-IVM provided a larger field-of-view with temporal resolution suitable for capturing initial fast immune cell interactions with the SC microvasculature [34, 38]. EPI-IVM technique was limited by its lower spatial resolution and an imaging depth of 70–100 μm. It also carries an appreciable risk for photobleaching/damage, which can require frequent disruptions of imaging times to minimize the risk, and may inadvertently hinder cellular tracking [11]. In contrast, 2P-IVM provided greater spatial resolution, at increased depths with negligible risk of photobleaching/damage. 2P-IVM imaging provided lower temporal resolution, which was suitable for slow moving immune cell interactions (diapedesis across endothelial wall) [11, 17, 32, 35]. 2P-IVM also afforded additional advanced techniques such as second-harmonic generation, which can be used to identify the depth of the pial compartment (pia in the SC has a high concentration of collagen) relative to the depth of imaging analysis [11], or fluorescence recovery after photobleaching, which can be used for evaluating specific cellular phenotypes [31]. Different in vivo imaging modalities can be combined to complement technique-specific limitations [12, 16].
Methods in the surgical preparation of the SC for IVM can vary based on the imaging modality used and SC region-of-interest. IVM of the SC requires a surgical window and leaving the dura intact can reduce the risk of unintended trauma, but can also limit imaging depth due to increased optical scattering. Recommendations to remove the dura and avoid interference by dural vasculature were specifically reported for EPI-IVM [11]. The lumbar SC carries less optical scattering and interference than the cervical SC due to decreased density of white matter tracts and microvasculature, making it easier to image the lumbar SC with IVM. It is only more recently that 2P-LSM techniques have been developed to evaluate the cervical SC [11]. Differences observed in the angioarchitecture and hemodynamic parameters of the cervical vs. lumbar SC suggest that immune cell/microvascular interactions may also vary [11].
Current IVM techniques are limited to the evaluation of the superficial layers of the dorsal SC. The dura, potential subdural space, arachnoid, subarachnoid-space, pia, and subpial-space (in continuity with perivascular space) [4, 41] should be clearly differentiated in future IVM investigations of SC microvasculature. A good/superb understanding of the anatomical arrangement of the microvasculature is required for the implementation of IVM. In general, penetrating arteries are surrounded by Virchow–Robin spaces along their course (CSF-containing space between pia and glia limitans formed by astrocytic endfeet), which then give rise to arterioles, and capillaries [4]. As arterioles branch into capillaries, the perivascular space is not present, making the capillary wall adjacent to the parenchyma. Capillaries can branch out several times, until they converge on postcapillary venules, where perivascular space is again present while draining into ascending veins. This compartmental organization explains how trans-vascular routes in peri-arteriole/venule regions need to cross the glia limitans to enter the parenchyma, or how the infusion of intrathecal fluorescent markers can tag perivascular antigen-presenting cells along the pial–glial basement membranes [17]. Variations of the compartmental spaces in the setting of pathology (inflammation, trauma, etc.) can hinder the identification of vascular segments in their appropriate compartments. Identifying cells in the pia–parenchymal interface vs. parenchyma can be facilitated by the removal of dura and/or leptomeninges [31]. Future studies are encouraged to distinguish the microvascular segments in the compartments evaluated [9]. Please see discussion in the Supplementary for additional details and references for further reading.
Modifications in surgical windows with increased ventrolateral access to the SC and the development of three-photon excited fluorescence microscopy hold promise to overcoming current imaging limitations in the SC [13]. Additional details on using 2P-IVM in mouse models of EAE [10, 11] or SCI [25], or employing a window-chamber for long-term imaging [12], imaging immune cell interactions [9], and other general IVM techniques [13] can be found in the referenced studies. Data analysis depends on a number of manual techniques to extract and quantify data from IVM (precluding the ability for blind assessment or randomization), and there is a need for more objective approaches and standardization.
BLI and FMT
BLI can be used to evaluate changes in the expression of an efflux transporter at the blood–CNS barriers through the use of a transgenic model that implemented transcription of human P-glycoprotein along with a luciferase reporter [39]. Efflux transporters serve a protective function for the CNS by the efflux of toxins; however, they are also involved in the mechanism of drug resistance by preventing the delivery of therapeutic agents to the parenchyma. The bioluminescence produced with systemic administration of D-luciferin has been shown to correlate with levels of P-glycoprotein at the endothelial barrier. Variabilities in the bioavailability of D-luciferin in the CNS can artificially decrease the degree of bioluminescence produced and confound outcomes [39]. In addition, BLI is limited by absorption and scatter of signal due to the depth of signal source, and is restricted to the production of two-dimensional data [26].
In contrast, fluorescent signals have the ability to penetrate more tissue with less attenuation by absorption, allowing increased resolution of deep signals and three-dimensional localization with tomographic imaging. FMT with NIRF can be used to assess BSCB permeability, and has been shown to correlate increased permeability with signs of neurologic disease in EAE mouse models [26]. BLI and FMT both have potential use in preclinical screening models (i.e., drug delivery/discovery) given their high-throughput screening capacity. Their major limitations are poor imaging resolution and signal specificity.
Clinical relevance
In vivo investigations of the BSCB in SC disease have identified and evaluated a number of potential therapeutic targets [5, 7, 8, 16, 17]. The development of anti–α4-integrin antibody (natalizumab), as an approved therapy for MS, was based on the discovery that α4-integrins play a significant role in T-cell invasion [4, 34]. While interventions can be beneficial during the exacerbation of the disease, the same approach may cause unintended exacerbations during recovery. Therefore, assessing the sequence of events that occur in BSCB dysfunction can elucidate the window of opportunity for therapeutic interventions [4]. Differences in human and animal microvasculature function can limit the potential clinical translation of findings (see Supplementary text for further discussion).
A number of these imaging techniques have direct clinical applicability. CE-MRI already plays a pivotal role in the diagnosing and monitoring of patients with MS [30]. The feasibility of DCE-MRI with pharmacokinetic modeling for analysis was shown in the evaluation of intradural spinal lesions [42]. The feasibility and reproducibility of DCE-CT in the healthy cervical SC has also been shown [43]. Information acquired with DCE techniques may have high prognostic value. Challenges in implementing DCE MR/CT techniques are similar to the obstacles faced with preclinical models, given small dimensions of the SC (diameter ~12–14 mm human vs. 3.5 mm rat or 1 mm mouse) and associated motion artifacts. The need to minimize radiation would be a specific additional constraint for DCE-CT.
Targeted molecular-MRI using alternative contrast agents (i.e., IOCA) has also been evaluated in pilot clinical prospective investigations and may provide earlier detection of disease onset prior to symptoms in patients with MS [30]. The use of IOCA conjugated with an antibody to select targets may further increase the sensitivity of MRI in detecting disease activity in MS [27]. These techniques hold potential for companion diagnosis, but their major limitation is the unknown clinical safety. IOCAs are at risk for reaching toxic concentrations due to the poor biodegradability in its current molecular structure, and would require further modifications to improve its safety profile for clinical use.
Conventional US imaging is currently utilized in clinical settings [36]. US holds potential to improve the monitoring and prognosis of patients with SCI, with intraoperative imaging during and immediately after surgical decompression. The major limitations would be its dependency for surgical access (direct imaging limited to intraoperative use) and lack of formalized investigations into the clinical significance of their use.
Fluorescein is a widely adopted technique in intraoperative angiographic techniques, and the feasibility of its use with intraoperative IVM in humans has been shown in evaluating the microvasculature of melanoma during surgical treatment to guide drug delivery and immunotherapy strategies [44]. This was also applicable in processing objective intraoperative measurements of cortical perfusion before and after complex cerebral revascularization, for intraoperative monitoring of treatment [45]. However, the feasibility of this approach in studying the microvasculature of the SC has not been shown. This technique could provide great utility in clinical research, i.e., microvascular assessment to obtain measurements as prognostic indicators or to aid targeted local therapeutic delivery in patients afflicted with SCI. The implementation of this technique will likely have similar hurdles as it has in preclinical models/techniques with challenges in depth limitation, and the lack of standardized methods in acquisition and analysis. Technique advancements and increasing preclinical IVM studies in SCI may promote its translational use in the SC within the next decade.
Limitations and additional considerations
The current study is limited by its design. The restriction to the English language literature carries a risk for language bias in the selection of studies. The inclusion of heterogeneous investigations limited the analysis to qualitative synthesis. Study quality analysis demonstrated potential evaluation bias in the included studies [15]. Study findings can vary based on preclinical model-type selections. Interspecies and intraspecies differences in pathological responses to SCI, including BSCB disruption patterns, have been shown [22, 23]. Contusion SCI models cause moderate-severe injury, with consistent reports of exacerbation extending both rostrally and caudally from the lesion site [15], whereas in other SCI models the injury remains localized the site of the insult [25], or results in less severe injury types [19]. Pharmacokinetic modeling of DCE-MRI studies was based on a moderate-severe contusion-type injury; therefore, other models require further investigations to validate appropriate pharmacokinetic models [21]. EAE model types can reproduce a number of patterns with different timing of clinical disease activity, i.e., induced with CNS-myelin peptides or adoptive transfer of myelin-specific T cells, resulting in relapsing-remitting or chronic-progressive disease courses, depending on species and strain [34, 35, 38], and were evaluated with respect to the timepoint of disease activity.
It is possible that not all eligible articles were captured. The terminology used to reference the SC microvascular barrier varied, as more than half of the studies referred to the BSCB as the BBB. The BSCB is located in a unique microenvironment, with different expression levels of barrier-specific proteins and permeability patterns [1,2,3]. When compared to the BBB, increased permeability to radiotracers and cytokines, and decreased expression of transporters, tight, and cell junction proteins have been observed in investigations of the BSCB. These differences may account for the unique physiology and propensity of pathologic insults that predominantly occur in the SC, i.e., a higher rate of tight-junction remodeling in the SC microvasculature and permeability to inflammatory cytokines may be a predisposition to EAE [1, 2]. The BSCB is relatively understudied compared to the BBB, and our current knowledge is limited [3]. Further studies are needed to elucidate the significance of these differences and potentially unravel others.
Conclusion
In vivo investigation of the BSCB can provide valuable information on interactions that drive pathological events in SC disease. However, the number of studies was limited, and they are focused on two disease models. Additional studies are needed in less commonly studied SC disorders, and to establish standardized protocols for data acquisition and analysis. Further development of the reported techniques and multimodal approaches could overcome current imaging restrictions in the SC. These advancements would promote higher quality studies, wider adoption, and could have greater potential for clinical translation.
References
Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol. 2011;70:194–206.
Lutz SE, Smith JR, Kim DH, Olson CVL, Ellefsen K, Bates JM, et al. Caveolin1 is required for Th1 cell infiltration, but not tight junction remodeling, at the blood-brain barrier in autoimmune neuroinflammation. Cell Rep. 2017;21:2104–17.
Sharma HS. Pathophysiology of the blood–spinal cord barrier in traumatic injury. In: Sharma HS, Westman J, editors. Blood-spinal cord and brain barriers in health and disease. San Diego, California: Elsevier Academic Press; 2004. p. 437–518.
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78.
Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227.
Bilgen M, Dogan B, Narayana PA. In vivo assessment of blood-spinal cord barrier permeability: serial dynamic contrast enhanced MRI of spinal cord injury. Magn Reson Imaging. 2002;20:337–41.
Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci. 2009;29:753–64.
Tang P, Zhang Y, Chen C, Ji X, Ju F, Liu X, et al. In vivo two-photon imaging of axonal dieback, blood flow, and calcium influx with methylprednisolone therapy after spinal cord injury. Sci Rep. 2015;5:9691.
Borjini N, Paouri E, Tognatta R, Akassoglou K, Davalos D. Imaging the dynamic interactions between immune cells and the neurovascular interface in the spinal cord. Exp Neurol. 2019;322:113046.
Davalos D, Lee JK, Smith WB, Brinkman B, Ellisman MH, Zheng B, et al. Stable in vivo imaging of densely populated glia, axons and blood vessels in the mouse spinal cord using two-photon microscopy. J Neurosci Methods. 2008;169:1–7.
Haghayegh Jahromi N, Tardent H, Enzmann G, Deutsch U, Kawakami N, Bittner S, et al. A novel cervical spinal cord window preparation allows for two-photon imaging of T-cell interactions with the cervical spinal cord microvasculature during experimental autoimmune encephalomyelitis. Front Immunol. 2017;8:406.
Figley SA, Chen Y, Maeda A, Conroy L, McMullen JD, Silver JI, et al. A spinal cord window chamber model for in vivo longitudinal multimodal optical and acoustic imaging in a murine model. PLoS One. 2013;8:e58081.
Cheng YT, Lett KM, Schaffer CB. Surgical preparations, labeling strategies, and optical techniques for cell-resolved, in vivo imaging in the mouse spinal cord. Exp Neurol. 2019;318:192–204.
Hooijmans CR, Ritskes-Hoitinga M. Progress in using systematic reviews of animal studies to improve translational research. PLoS Med. 2013;10:e1001482. https://doi.org/10.1371/journal.pmed.1001482.
Watzlawick R, Antonic A, Sena ES, Kopp MA, Rind J, Dirnagl U, et al. Outcome heterogeneity and bias in acute experimental spinal cord injury: a meta-analysis. Neurology. 2019;93:e40–51.
Aube B, Levesque SA, Pare A, Chamma E, Kebir H, Gorina R, et al. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol. 2014;193:2438–54.
Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–8.
Bendszus M, Ladewig G, Jestaedt L, Misselwitz B, Solymosi L, Toyka K, et al. Gadofluorine M enhancement allows more sensitive detection of inflammatory CNS lesions than T2-w imaging: a quantitative MRI study. Brain. 2008;131:2341–52.
Berens SA, Colvin DC, Yu CG, Yezierski RP, Mareci TH. Evaluation of the pathologic characteristics of excitotoxic spinal cord injury with MR imaging. AJNR Am J Neuroradiol. 2005;26:1612–22.
Bilgen M, Abbe R, Narayana PA. Dynamic contrast-enhanced MRI of experimental spinal cord injury: in vivo serial studies. Magn Reson Med. 2001;45:614–22.
Bilgen M, Narayana PA. A pharmacokinetic model for quantitative evaluation of spinal cord injury with dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Med. 2001;46:1099–106.
Byrnes KR, Fricke ST, Faden AI. Neuropathological differences between rats and mice after spinal cord injury. J Magn Reson Imaging. 2010;32:836–46.
Cahill LS, Laliberte CL, Liu XJ, Bishop J, Nieman BJ, Mogil JS, et al. Quantifying blood-spinal cord barrier permeability after peripheral nerve injury in the living mouse. Mol Pain. 2014;10:60.
Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu SJ, et al. Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed. 2009;22:332–41.
Dray C, Rougon G, Debarbieux F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc Natl Acad Sci USA. 2009;106:9459–64.
Eaton VL, Vasquez KO, Goings GE, Hunter ZN, Peterson JD, Miller SD. Optical tomographic imaging of near infrared imaging agents quantifies disease severity and immunomodulation of experimental autoimmune encephalomyelitis in vivo. J Neuroinflammation. 2013;10:138.
Fournier AP, Quenault A, Martinez de Lizarrondo S, Gauberti M, Defer G, Vivien D, et al. Prediction of disease activity in models of multiple sclerosis by molecular magnetic resonance imaging of P-selectin. Proc Natl Acad Sci USA. 2017;114:6116–21.
Herrera JJ, Sundberg LM, Zentilin L, Giacca M, Narayana PA. Sustained expression of vascular endothelial growth factor and angiopoietin-1 improves blood-spinal cord barrier integrity and functional recovery after spinal cord injury. J Neurotrauma. 2010;27:2067–76.
Kang CE, Clarkson R, Tator CH, Yeung IW, Shoichet MS. Spinal cord blood flow and blood vessel permeability measured by dynamic computed tomography imaging in rats after localized delivery of fibroblast growth factor. J Neurotrauma. 2010;27:2041–53.
Ladewig G, Jestaedt L, Misselwitz B, Solymosi L, Toyka K, Bendszus M, et al. Spatial diversity of blood-brain barrier alteration and macrophage invasion in experimental autoimmune encephalomyelitis: a comparative MRI study. Exp Neurol. 2009;220:207–11.
Locatelli G, Theodorou D, Kendirli A, Jordao MJC, Staszewski O, Phulphagar K, et al. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci. 2018;21:1196–208.
Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schlager C, Lodygin D, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–9.
Patel CB, Cohen DM, Ahobila-Vajjula P, Sundberg LM, Chacko T, Narayana PA. Effect of VEGF treatment on the blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced magnetic resonance imaging. J Neurotrauma. 2009;26:1005–16.
Sathiyanadan K, Coisne C, Enzmann G, Deutsch U, Engelhardt B. PSGL-1 and E/P-selectins are essential for T-cell rolling in inflamed CNS microvessels but dispensable for initiation of EAE. Eur J Immunol. 2014;44:2287–94.
Schellenberg AE, Buist R, Yong VW, Del Bigio MR, Peeling J. Magnetic resonance imaging of blood-spinal cord barrier disruption in mice with experimental autoimmune encephalomyelitis. Magn Reson Med. 2007;58:298–305.
Soubeyrand M, Badner A, Vawda R, Chung YS, Fehlings MG. Very high resolution ultrasound imaging for real-time quantitative visualization of vascular disruption after spinal cord injury. J Neurotrauma. 2014;31:1767–75.
Tatar I, Chou PC, Desouki MM, El Sayed H, Bilgen M. Evaluating regional blood spinal cord barrier dysfunction following spinal cord injury using longitudinal dynamic contrast-enhanced MRI. BMC Med Imaging. 2009;9:10.
Vajkoczy P, Laschinger M, Engelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–65.
Yasuda K, Cline C, Lin YS, Scheib R, Ganguly S, Thirumaran RK, et al. In vivo imaging of human MDR1 transcription in the brain and spine of MDR1-luciferase reporter mice. Drug Metab Dispos. 2015;43:1646–54.
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–32.
Weller RO, Sharp MM, Christodoulides M, Carare RO, Mollgard K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 2018;135:363–85.
Cuvinciuc V, Viallon M, Barnaure I, Vargas MI, Lovblad KO, Haller S. Dynamic contrast-enhanced MR perfusion of intradural spinal lesions. AJNR Am J Neuroradiol. 2017;38:192–4.
Bisdas S, Rumboldt Z, Surlan K, Koh TS, Deveikis J, Spampinato MV. Perfusion CT measurements in healthy cervical spinal cord: feasibility and repeatability of the study as well as interchangeability of the perfusion estimates using two commercially available software packages. Eur Radiol. 2008;18:2321–8.
Fisher DT, Muhitch JB, Kim M, Doyen KC, Bogner PN, Evans SS, et al. Intraoperative intravital microscopy permits the study of human tumour vessels. Nat Commun. 2016;7:10684.
Rennert RC, Strickland BA, Ravina K, Bakhsheshian J, Fredrickson V, Carey J, et al. Intraoperative assessment of cortical perfusion after intracranial-to-intracranial and extracranial-to-intracranial bypass for complex cerebral aneurysms using Flow 800. Oper Neurosurg (Hagerstown). 2019;16:583–92.
Funding
JB and BAS were funded by NIH NINDS R25NS099008 USC Neurosurgery Research Education Training Program. WJM was funded by R01ES024936, P01AG055367 Sub 5202, and R25NS099008. BVZ was funded by R01AG039452, R01NS100459, R01AG023084, R01NS090904, and R01NS034467.
Author information
Authors and Affiliations
Contributions
JB, WJM, and BVZ were responsible for designing the review protocol. JB and BAS were responsible for conducting the search, screening potentially eligible studies, extracting and analyzing data, interpreting results, creating tables, and writing the report. WJM and BVZ were responsible for arbitrating potentially eligible studies, and interpreting results, and provided feedback on the final report.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Systematic review registration number: PROSPERO (CRD42020183871)
Supplementary information
Rights and permissions
About this article
Cite this article
Bakhsheshian, J., Strickland, B.A., Mack, W.J. et al. Investigating the blood–spinal cord barrier in preclinical models: a systematic review of in vivo imaging techniques. Spinal Cord 59, 596–612 (2021). https://doi.org/10.1038/s41393-021-00623-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00623-7