Abstract
Background
Waist-to-height ratio (WHtR) predicts abdominal fat and cardiometabolic risk. In children with obesity, the most adequate cut-off to predict cardiometabolic risk as well as its ability to predict risk changes over time has not been tested. Our aim was to define an appropriate WHtR cut-off to predict cardiometabolic risk in children with obesity, and to analyze its ability to predict changes in cardiometabolic risk over time.
Methods
This is an observational prospective study secondary to the OBEMAT2.0 trial. We included data from 218 participants (8–15 years) who attended baseline and final visits (12 months later). The main outcome measure was a cardiometabolic risk score derived from blood pressure, lipoproteins, and HOMA index of insulin resistance.
Results
The optimal cut-off to predict the cardiometabolic risk score was WHtR ≥0.55 with an area under the curve of 0.675 (95% CI: 0.589–0.760) at baseline and 0.682 (95% CI: 0.585–0.779) at the final visit. Multivariate models for repeated measures showed that changes in cardiometabolic risk were significantly associated with changes in WHtR.
Conclusion
This study confirms the clinical utility of WHtR to predict changes in cardiometabolic risk over time in children with obesity. The most accurate cut-off to predict cardiometabolic risk in children with obesity was WHtR ≥0.55.
Impact
-
In children, there is no consensus on a unique WHtR cut-off to predict cardiometabolic risk.
-
The present work provides sufficient evidence to support the use of the 0.55 boundary.
-
We have a large sample of children with obesity, with whom we compared the previously proposed boundaries according to cardiometabolic risk, and we found the optimal WHtR cut-off to predict it.
-
We also analyzed if a reduction in the WHtR was associated with an improvement in their cardiometabolic profile.
Similar content being viewed by others
Introduction
In obesity, the excess fat mass accumulation in the subcutaneous tissue leads to an increase in circulating free fatty acids. These free fatty acids could lay down in other fat depots such as visceral adipose tissue, or infiltrate the liver, muscle, pancreas, heart, or kidney and thus alter their metabolic function.1,2 The distribution of the adipose tissue is strongly related to the cardiometabolic risk. Large accumulations of visceral and ectopic adipose tissue are related to the development of several pathologies like type 2 diabetes mellitus, dyslipidemia, hypertension, cardiovascular diseases, non-alcoholic fatty liver disease, or some types of cancer.3 Several studies to date confirmed that there is a large tracking of adiposity between childhood and adulthood,4 and childhood overweight and obesity are associated with increased cardiovascular events in adulthood.5
Several techniques can be used to quantify fat mass (air displacement plethysmography, dual-energy-X-ray absorptiometry, magnetic resonance imaging, or computed tomography) but most of them are expensive, or do not distinguish between central and peripheral fat mass, or are not portable or require radiation exposure.6,7 Waist circumference (WC) is an indicator of visceral adipose tissue,8 which has been significantly associated with cardiometabolic comorbidities in children and adolescents.9 It is easy to perform, low-cost, and widely used in adults to predict cardiovascular risk. In adults, there are specific cut-off points for WC by sex (>88 cm in women and >102 cm in men)10 that predict cardiovascular risk. In children and adolescents, as they are growing, there is not a universal cut-off and it is necessary to consult specific reference values.11 Waist-to-height ratio (WHtR) has been proposed as a simpler method as a proxy for abdominal obesity in the pediatric population since it is independent of age and sex and can be interpreted according to a fixed cut-off point in clinical practice.12 It has been suggested that WHtR could be a better predictor of abdominal adiposity than WC.13 WHtR is strongly associated with the visceral fat measured using imaging techniques,14 has been related to cardiometabolic risk factors15,16 and increased risk of metabolic syndrome17 in children and adolescents. In the last several decades, there was a trend to increase in obesity prevalence in children and adolescents.18 Especially, there has been a greater proportional increase in WC, and therefore in abdominal obesity, than in BMI in adolescents.19 Actually, several studies reported that WHtR assessed cardiometabolic risk in children better than BMI.16,20 In adults, a boundary value of 0.50 has been established to indicate cardiometabolic risk, in accordance with the public health message “keep your WC to less than half your height”.21 In children, there is not a unique cut-off internationally accepted for WHtR. Many authors support the use of WHtR ≥0.50 cut-off as well to assess cardiometabolic risk in children12,15,21,22,23,24,25,26,27 even though several of them obtained a low specificity, leading to a high proportion of false positives. Other authors have suggested higher boundary values. Khoury et al.28 using data from the National Health and Nutrition Examination Survey, classified children into three groups of WHtR: ≥0.50, ≥0.55, and ≥0.60. Children with WHtR ≥0.60 had an increased prevalence of cardiometabolic risk factors compared to the other groups. Ochoa Sangrador et al.17, in a systematic review in 2018 showed that the WHtR cut-offs proposed for the pediatric population ranged from 0.40 to 0.55. Those cut-off points above 0.50 were predictors of a higher risk of metabolic syndrome. Recently, we published that a WHtR ≥0.55 seemed to be the most appropriate boundary value in children from the general population from five different European countries.16
The aim of the present work was to define an appropriate fixed cut-off to diagnose abdominal obesity in children with obesity aged 8–15 years, by comparing the sensitivity, specificity, and predictive capacity of three different WHtR cut-offs: WHtR ≥0.50, WHtR ≥0.55, and WHtR ≥0.60 for predicting cardiometabolic risk factors. Furthermore, to confirm its clinical utility, we aimed to evaluate whether changes in WHtR associated with an intervention were also accompanied by an improvement in the cardiometabolic profile.
Methods
Design
This was an observational longitudinal prospective study secondary to the OBEMAT2.0 trial (formerly a randomized clustered clinical trial with the clinical.gov identifier NCT02889406). Briefly, OBEMAT2.0 study evaluated the clinical and metabolic efficacy of the coordinated motivational approach between primary and specialized care for the treatment of childhood obesity. Children were treated in one of the clusters for 12 (+3) months: a control group following the usual recommendations in primary care and an intervention group receiving motivational therapy including family education workshops on nutrition, healthy cooking techniques, and physical activity supported by educational materials and eHealth (wearable). Further details of the clinical trial were previously published.29
Study population
Children between 8 and <15 years at recruitment, and diagnosed of childhood obesity according to the Guidelines for Clinical Practice on the Prevention and Treatment of Childhood and Adolescent Obesity of the Spanish National Health System30 were enrolled in the study in their primary care centers during regular health checks. Children with eating disorders or presence of endocrine disorders were excluded. The original sample size calculation to participate in the clinical trial was detailed in the original protocol of the clinical trial.29
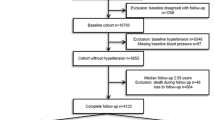
Only children with full data set were included in the analyses (children with missing data for the main health outcomes predictors were not included). In the present study, we included data from 218 children (independently of the adherence to the intervention), who attended the baseline (from June 2016 to March 2018) and the final (from June 2017 to June 2019) assessment visits in their reference hospital (University Hospital Sant Joan de Reus or University Hospital Joan XXIII de Tarragona), from the 315 recruited in the OBEMAT2.0 clinical trial. The flow diagram of participants is shown in Supplementary Fig. 1.
Data collection
The WHtR was considered as the diagnostic test and different altered cardiometabolic risk parameters were considered as the target disease condition. All these measures, detailed below, were taken during the same day, both baseline and final visits.
Anthropometry
All anthropometric measures were taken by the same trained personnel at baseline and final study visits. Weight (kg) and height (m) were measured using a SECA769 scale (precision 50 g), SECA 216 Stadiometer (precision 1 mm). WC (cm) was measured at the mid-point between the iliac crest and the lower rib with a Holtain WC non-extensible tape (precision 1 mm). BMI (kg/m2) was calculated as weight (kg)/height (m2) and its z-score (BMI z-score) for age and sex according to the World Health Organization references.31 WHtR was calculated as WHtR = WC (cm)/height (cm), and all the participants were categorized into groups according to the three boundary values for abdominal obesity: WHtR ≥0.50, WHtR ≥0.55, and WHtR ≥0.60.
Blood pressure
At baseline and final visits, study personnel measured systolic (SBP) and diastolic (DBP) blood pressure (mmHg) using a Dinamap Pro 100 device, with the most adequate cuff size for each participant. Blood pressure was measured at least 20 min after arriving at the study center in duplicate, with an interval of 5 min between measures in the left arm, while the child remained sat down with the arm resting comfortably. The mean of both measures was calculated and used for all subsequent analyses.
SBP and DBP variables were categorized into z-scores according to Stavnsbo et al.32, considering altered a z-score ≥1.5 SD for age and sex.
Blood sample
At baseline and final visits, a blood sample was drawn from children during overnight fasting. Glucose (mg/dL), insulin (mIU), total cholesterol (mg/dL), high-density lipoprotein cholesterol (HDL) (mg/dL), low-density lipoprotein cholesterol (LDL) (mg/dL), and triglycerides (mg/dL) were analyzed in the laboratories of the reference hospitals. Insulin resistance index (HOMA-IR) was calculated from insulin and glucose as HOMA-IR = (Insulin (µU/mL) × Glucose (mmol/L)) / 22.5.
The HDL cholesterol, triglycerides, and HOMA-IR z-scores were calculated according to Stavnsbo et al.32, considering as altered for triglycerides and HOMA-IR a z-score ≥1.5 SD for age and sex and for HDL cholesterol a z-score ≤1.5 SD.
Assessment of cardiometabolic risk
To assess the children’s cardiometabolic risk, we created a continuous cardiometabolic risk score (Cmet Risk) based on Eisenmann et al.33. This score was the sum of the standardized SBP, DBP, triglycerides, HOMA-IR, and HDL cholesterol z-scores, this last one multiplied by –1 (as HDL cholesterol is inversely related to cardiometabolic risk). A higher score was indicative of a less favorable cardiometabolic profile. It is worth highlighting that for the definition of cardiometabolic risk score, we excluded the WC (which is usually considered in metabolic syndrome), because it was part of our predicting variable. A child with the presence of two or more altered health outcomes (SBP, DBP, triglycerides, or HOMA-IR ≥1.5 SD and/or HDL cholesterol ≤1.5 SD for age and sex) was considered to have an altered cardiometabolic risk.
Statistics
The description of the continuous variables is presented either as mean and standard deviation or median and interquartile range (25th–75th percentiles) as appropriate. Normality was verified using the Kolmogorov–Smirnov test. Differences between baseline and final visits were assessed by either Student t-tests for repeated measures or Wilcoxon tests depending on the distribution of variables.
Mann–Whitney U tests were used to compare the medians of biochemical parameters and blood pressure according to the different abdominal obesity categories.
Logistic binary regression analyses were performed to quantify the odds of having health risk factors and cardiometabolic risk by different abdominal obesity cut-offs (WHtR ≥0.50, WHtR ≥0.55, and WHtR ≥0.60). Models were adjusted by sex and age.
We calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the three cut-offs for predicting cardiometabolic risk. In addition, we conducted receiver operating characteristic (ROC) analyses to determine the area under the curve (AUC) of the WHtR, as well as the optimal cut-off to predict cardiometabolic risk.
To assess whether the longitudinal change in cardiometabolic risk factors between visits was associated to changes in WHtR, general linear models for repeated measures were performed, adjusted by sex and age.
Statistical significance was accepted at the level p < 0.05. Statistical analyses were carried out with the software package SPSS Statistics version 27.0 (SPSS Inc., Chicago, IL).
Results
Two hundred eighteen children (119 boys, 55% boys) who attended baseline and final visits were included in the analysis. Table 1 shows the anthropometrics and biochemical characteristics of the participants and their comparison before and after the intervention.
At baseline visit, 95, 71, and 22 of the children had a WHtR ≥0.50, a WHtR ≥0.55, and a WHtR ≥0.60, respectively. At final visit, 86, 52, and 17% of the children had a WHtR ≥0.50, a WHtR ≥0.55, and a WHtR ≥0.60, respectively. Tracking of abdominal obesity categories according to the different boundaries was as follows: 11% of the children who were ≥0.50 cut-off at baseline changed to <0.50 at the end of the intervention; 31 and 47% of the children that were equal or above the 0.55 and 0.60 cut-offs, respectively, at baseline visit were under this at the final one. The distribution of the prevalence of altered health risk factors and the presence of cardiometabolic risk according to the different abdominal obesity cut-offs at baseline and final visits is shown in Supplementary Table 1.
Table 2 shows the descriptive and comparison of cardiometabolic risk parameters according to having or not having abdominal obesity by the different boundaries (WHtR ≥0.50 vs. WHtR <0.50, WHtR ≥0.55 vs. WHtR <0.55 and WHtR ≥0.60 vs. WHtR <0.60) at baseline and final visits.
At baseline visit, cardiometabolic risk parameters were similar in children with and without abdominal obesity according to the 0.5 and 0.55 criteria. However, children with a WHtR ≥0.60 showed a statistically significant worse cardiometabolic profile compared to those below the cut-off. At final visit, the three boundaries showed significant differences between having or not abdominal obesity for HDL cholesterol, triglycerides, HOMA-IR, and the cardiometabolic risk score, and only with the 0.55 cut-off for DBP.
The results of the binary logistic regression analysis for baseline and final visits are shown in Table 3. Children with abdominal obesity did not differ in any of the cardiometabolic risk parameters from children without abdominal obesity according to the WHtR ≥0.50 boundary, at any of the visits. Children with abdominal obesity according to the ≥0.55 boundary had significantly higher odds of high DBP, triglycerides, HOMA-IR, and overall cardiometabolic risk compared to children without abdominal obesity at final visit. Children with abdominal obesity according to the ≥0.60 boundary had significantly higher odds of low HDL cholesterol and high HOMA-IR and cardiometabolic risk at baseline, and higher odds of elevated HOMA-IR and cardiometabolic risk at final visit compared to children without obesity according to the WHtR <0.60 criteria.
We calculated the sensitivity, specificity, PPV, and NPV for the three boundaries in distinguishing subjects with cardiometabolic risk at both visits (Table 4). WHtR ≥0.50 and WHtR ≥0.55 showed higher sensitivity values (ranging from 82 to 97%); however, WHtR ≥0.60 provided the most elevated specificity in both time points (82 and 87%). Despite this, all of them had low PPV but high NPV. ROC curve was also performed to find the optimal WHtR to predict cardiometabolic risk at baseline and final visits. At baseline, the AUC was 0.675 and the optimal cut-off was 0.5609, with a sensitivity and specificity of 85 and 46%, respectively. At final visit, the AUC was 0.682 and the most suitable cut-off was 0.5516, with a sensitivity and specificity of 82 and 59%, respectively (Fig. 1). These tests were also performed separately by sex (Supplementary Table 2 and Supplementary Fig. 2), with similar cut-offs, although with lower sensitivity among girls.
Non-adjusted analyses revealed that variation in WHtR between visits (baseline and final) was significantly but weakly correlated with the change in all biochemical measurements (HDL cholesterol, triglycerides, HOMA-IR) and the cardiometabolic risk: r = 0.168 (p = 0.017); r = 0.204 (p = 0.003); r = 0.197 (p = 0.006) and r = 0.217 (p = 0.004) for HDL cholesterol, triglycerides, HOMA-IR and cardiometabolic risk, respectively. Increments in WHtR between visits were not associated with changes in SBP or DBP between visits. Table 5 shows the results of the general linear model for repeated measures for changes in cardiometabolic risk parameters associated with increments in WHtR. Adjusted models showed a significant interaction between WHtR and biochemical parameters (HDL cholesterol, triglycerides, HOMA-IR) and the cardiometabolic risk, which revealed that the changes in those parameters between visits were associated with changes in WHtR. Consistently with simple correlation analyses, SBP and DBP did not show any association with changes in WHtR in adjusted models for repeated measures.
Discussion
This study has shown the clinical usefulness of using WHtR and the different boundaries in children and adolescents with obesity to predict cardiometabolic risk.
In our sample, although all children had obesity according to BMI, we found statistically significant differences in cardiometabolic risk factors and overall cardiometabolic risk between children with abdominal obesity vs. without abdominal obesity for the three boundaries. This is in accordance with data from the Bogalusa Heart Study,22 in which children with obesity and normal WHtR (<0.50) had significantly lower levels of cardiometabolic risk factors compared to those with obesity and elevated WHtR (≥0.50). These findings evidence that not all individuals with generalized obesity have the same health risk, and support the use of a measure of abdominal obesity (either WC or WHtR) in the definition of metabolic syndrome rather than BMI.34,35,36 Following this, the concept of distinguishing “metabolically healthy obesity” (in which despite the presence of obesity, no metabolic abnormalities such as insulin resistance, dyslipidemia, or hypertension are present) from “metabolically unhealthy obesity” (for subjects with obesity accompanied by metabolic abnormalities)37,38 has recently gained interest. Genovesi et al.39 found that WHtR was an independent predictor of developing metabolically unhealthy obesity.
Several studies proposed different cut-offs of WHtR to diagnose overweight and obesity in children by comparison with BMI or body composition that ranged between 0.45 and 0.54.40,41,42,43 However, some of these studies did not associate those cut-offs with cardiometabolic risk factors. When focusing on the most appropriate cut-offs of WHtR to be used in clinical practice, decisions should be taken according to the capacity to predict cardiometabolic risk, beyond the pure anthropometrical measure. In this line, several studies have explored appropriate WHtR cut-off points to identify cardiometabolic risk, and differed in their conclusions. Some authors supported the use of WHtR ≥0.50 as the most appropriate cut-off point to predict cardiometabolic risk21,22,23,44 in a wide age range (4–19 years old). Some authors found that even lower cut-off points would be useful to identify cardiometabolic risk in young children (5–9 years old).45 Arellano et al.46 proposed as the best predictor of cardiometabolic risk a WHtR ≥0.51 cut-off in children aged 8–11. Vasquez et al.47 found that a WHtR value of 0.54 was a good predictor of cardiometabolic risk in 16 years old children. In study samples with a higher percentage of obesity, the optimal cut-offs ranged between 0.55 and 0.6048,49,50,51 in children and adolescents (6–16 years old). Potential factors modifying the optimal boundary of WHtR are the age of the sample and the prevalence of generalized obesity that affect in turn the appearance of cardiometabolic alterations.
We found that the 0.50 cut-off (which is widely used in adults) was not a good predictor of cardiometabolic risk in children and adolescents with obesity. This is not surprising, since most of the children, whether they had metabolic alterations or not, due to having generalized obesity, were already above the 0.50 cut-off.
As we could see, the most frequently proposed cut-off for the general pediatric population was a value of 0.50, but when the prevalence of obesity increases, to differentiate those children who have a real cardiometabolic risk from those who do not, higher cut-off points are needed.
In our study, with the 0.55 and 0.60 cut-off values, the sensitivity and specificity to predict cardiometabolic risk were balanced, and had high PPV and NPV to discriminate cardiometabolic alterations among children with obesity.
In our study, the AUC to find the optimal cut-off was not very high. However, considering that this is a fast, innocuous screening tool that could avoid or optimize additional testing on children, an AUC near 0.7 might be acceptable. At the baseline visit, the best predictor of cardiometabolic risk was 0.56 and at the final visit 0.55, with adequate sensitivity and specificity. Our results are in accordance with those obtained by Arnaiz et al.48
Even, in a sample of European children with a prevalence of obesity <10%, we found that a WHtR ≥0.55 was more specific and identified better children with cardiometabolic risk, especially at younger ages.16
We can confirm the clinical utility of WHtR, as we have seen that the reduction of this parameter is associated with an improvement in cardiometabolic risk in children and adolescents with obesity. Our results agree with those of Kalavainen et al.52 who found a reduction in cardiometabolic risk and WHtR in children after a 6-month healthy habits intervention. Other studies showed significant decreases in WHtR after receiving lifestyle interventions but without relating it to cardiometabolic markers.53,54,55
We acknowledge that this study had some limitations: the sample size did not allow us to stratify the children by age, ethnic groups, or pubertal development, and the values of some cardiometabolic risk indicators may differ between pubertal stages. Although the study personnel were trained and followed standard procedures we cannot discard a potential source of bias from inter- and intra-variability in anthropometrical measurements. This study also has some strengths: We had a considerably large sample of children and adolescents diagnosed with obesity, which allowed us to see that although all of them had general obesity, the cardiometabolic risk varied depending on the abdominal obesity. Furthermore, the prospective longitudinal design of our study, with a longer intervention period has allowed us to analyze the impact of the reduction of WHtR on cardiometabolic risk. Considering the large sample size, the longitudinal design, and the consistency with previous publications we consider that our results could be generalized and applicable to the pediatric population with obesity.
Conclusion
The WHtR is a good predictor of cardiometabolic risk in children and adolescents with obesity. In our sample of children and adolescents with obesity, the WHtR cut-off point with better sensitivity and specificity to predict cardiometabolic risk was 0.55. The 0.50 cut-off, widely used in adults, was not useful to distinguish between children and adolescents with or without cardiometabolic risk. Thus, WHtR ≥0.55 could be considered an appropriate cut-off for abdominal obesity in children.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to their containing personal information but are available from the corresponding author on reasonable request.
References
Avolio, E. et al. Obesity and body composition in man and woman: associated diseases and the new role of gut microbiota. Curr. Med. Chem. 27, 216–229 (2019).
Bays, H. Adiposopathy, ‘sick fat,’ Ockham’s razor, and resolution of the obesity paradox. Curr. Atheroscler. Rep. 16, 409 (2014).
Borga, M. et al. Advanced body composition assessment: from body mass index to body composition profiling. J. Investig. Med. 66, 887–895 (2018).
Juonala, M. et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 365, 1876–1885 (2011).
Baker, J. L., Olsen, L. W. & Sørensen, T. I. A. Childhood body-mass index and the risk of coronary heart disease in adulthood. N. Engl. J. Med. 357, 2329–2337 (2007).
Andreoli, A., Garaci, F., Cafarelli, F. P. & Guglielmi, G. Body composition in clinical practice. Eur. J. Radiol. 85, 1461–1468 (2016).
Lemos, T. & Gallagher, D. Current body composition measurement techniques. Curr. Opin. Endocrinol. Diabetes Obes. 24, 310–314 (2017).
Trandafir, L. M. et al. Waist circumference a clinical criterion for prediction of cardio-vascular complications in children and adolescences with overweight and obesity. Medicine (Baltimore) 99, e20923 (2020).
Lee, S., Kuk, J. L., Boesch, C. & Arslanian, S. Waist circumference is associated with liver fat in black and white adolescents. Appl. Physiol. Nutr. Metab. 42, 829–833 (2017).
De Santis Filgueiras, M. et al. Waist circumference, waist-to-height ratio and conicity index to evaluate android fat excess in Brazilian children. Public Health Nutr. 22, 140–146 (2019).
López-González, D. et al. Diagnostic performance of waist circumference measurments for predicting cardiometabolic risk in Mexican children. Endocr. Pract. 22, 1170–1176 (2016).
Aguilar-Morales, I., Colin-Ramirez, E., Rivera-Mancía, S., Vallejo, M. & Vázquez-Antona, C. Performance of waist-to-height ratio, waist circumference, and body mass index in discriminating cardio-metabolic risk factors in a sample of school-aged Mexican children. Nutrients 10, 1850 (2018).
Ashwell, M., Cole, T. J. & Dixon, A. K. Ratio of waist circumference to height is strong predictor of intra-abdominal fat. Br. Med. J. 313, 559–560 (1996).
Soto González, A. et al. Predictors of the metabolic syndrome and correlation with computed axial tomography. Nutrition 23, 36–45 (2007).
Schröder, H. et al. Prevalence of abdominal obesity in Spanish children and adolescents. do we need waist circumference measurements in pediatric practice? PLoS One 9, 5–10 (2014).
Muñoz-Hernando, J. et al. Usefulness of the waist-to-height ratio for predicting cardiometabolic risk in children and its suggested boundary values. Clin. Nutr. 41, 508–516. https://doi.org/10.1016/j.clnu.2021.12.008 (2022).
Ochoa Sangrador, C. & Ochoa-Brezmes, J. Waist-to-height ratio as a risk marker for metabolic syndrome in childhood. A meta-analysis. Pediatr. Obes. 13, 421–432 (2018).
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
Mindell, J. S., Dinsdale, H., Ridler, C. & Rutter, H. R. Changes in waist circumference among adolescents in England from 1977-1987 to 2005-2007. Public Health 126, 695–701 (2012).
Lindholm, A. et al. Body mass index classification misses to identify children with an elevated waist-to-height ratio at 5 years of age. Pediatr. Res. 85, 30–35 (2019).
Browning, L. M., Hsieh, S. D. & Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 05 could be a suitable global boundary value. Nutr. Res. Rev. 23, 247–269 (2010).
Mokha, J. S. et al. Utility of waist-to-height ratio in assessing the status of central obesity and related cardiometabolic risk profile among normal weight and overweight/obese children: The Bogalusa Heart Study. BMC Pediatr. 10, 73 (2010).
Chung, I. H., Park, S., Park, M. J. & Yoo, E. G. Waist-to-height ratio as an index for cardiometabolic risk in adolescents: results from the 1998−2008 KNHANES. Yonsei Med. J. 57, 658–663 (2016).
Goulding, A. et al. Waist-to-height ratios in relation to BMI z-scores in three ethnic groups from a representative sample of New Zealand children aged 5-14 years. Int. J. Obes. 34, 1188–1190 (2010).
McCarthy, H. D. & Ashwell, M. A study of central fatness using waist-to-height ratios in UK children and adolescents over two decades supports the simple message - ‘keep your waist circumference to less than half your height’. Int. J. Obes. 30, 988–992 (2006).
Campagnolo, P. D. B., Hoffman, D. J. & Vitolo, M. R. Waist-to-height ratio as a screening tool for children with risk factors for cardiovascular disease. Ann. Hum. Biol. 38, 265–270 (2011).
Lopez-Legarrea, P. et al. Waist circumference and waist:height ratio percentiles using LMS method in Chilean population. Nutr. Metab. Cardiovasc. Dis. 27, 183–189 (2017).
Khoury, M., Manlhiot, C. & McCrindle, B. W. Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. J. Am. Coll. Cardiol. 62, 742–751 (2013).
Luque, V. et al. The OBEMAT2.0 study: a clinical trial of a motivational intervention for childhood obesity treatment. Nutrients 11, 419 (2019).
De, G. et al. Guía de Práctica Clínica sobre la Prevención y el Tratamiento de la Obesidad Infantojuvenil (Ministerio de Ciencia e Innovación [Spanish Ministry of Sciencee and Innovation], 2009).
De Onis, M. et al. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 85, 660–667 (2007).
Stavnsbo, M. et al. Reference values for cardiometabolic risk scores in children and adolescents: suggesting a common standard. Atherosclerosis 278, 299–306 (2018).
Eisenmann, J. C. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc. Diabetol. 7, 1–6 (2008).
Weihe, P. & Weihrauch-Blüher, S. Metabolic syndrome in children and adolescents: diagnostic criteria, therapeutic options and perspectives. Curr. Obes. Rep. 8, 472–479 (2019).
Nambiar, S., Truby, H., Davies, P. S. & Kimberley, B. Use of the waist-height ratio to predict metabolic syndrome in obese children and adolescents. J. Paediatr. Child Health 49, E281–E287 (2013).
Nambiar, S., Hughes, I. & Davies, P. S. Developing waist-to-height ratio cut-offs to define overweight and obesity in children and adolescents. Public Health Nutr. 13, 1566–1574 (2010).
Iacobini, C., Pugliese, G., Blasetti Fantauzzi, C., Federici, M. & Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism 92, 51–60 (2019).
Bosello, O., Donataccio, M. P. & Cuzzolaro, M. Obesity or obesities? Controversies on the association between body mass index and premature mortality. Eat. Weight Disord. 21, 165–174 (2016).
Genovesi, S. et al. Cardiovascular risk factors associated with the metabolically healthy obese (MHO) phenotype compared to the metabolically unhealthy obese (MUO) phenotype in children. Front. Endocrinol. (Lausanne). 11, 27 (2020).
Kahn, H. S., Imperatore, G. & Cheng, Y. J. A population-based comparison of BMI percentiles and waist-to-height ratio for identifying cardiovascular risk in youth. J. Pediatr. 146, 482–488 (2005).
Marrodán, M. et al. [Diagnostic accuracy of waist to height ratio in screening of overweight and infant obesity]. Med. Clin. (Barc.). 140, 296–301 (2013).
Weili, Y. et al. Waist-to-height ratio is an accurate and easier index for evaluating obesity in children and adolescents. Obesity (Silver Spring) 15, 748–752 (2007).
Asif, M., Aslam, M. & Altaf, S. Evaluation of anthropometric parameters of central obesity in Pakistani children aged 5-12 years, using receiver operating characteristic (ROC) analysis. J. Pediatr. Endocrinol. Metab. 31, 971–977 (2018).
Buchan, D. S. & Baker, J. Utility of body mass index, waist-to-height-ratio and cardiorespiratory fitness thresholds for identifying cardiometabolic risk in 10.4-17.6-year-old children. Obes. Res. Clin. Pract. 11, 567–575 (2017).
Graves, L. et al. Waist-to-height ratio and cardiometabolic risk factors in adolescence: findings from a prospective birth cohort. Pediatr. Obes. 9, 327–338 (2014).
Arellano-ruiz, P. et al. Predictive ability of waist circumference and waist-to-height ratio for cardiometabolic risk screening among Spanish children. Nutrients 12, 415 (2020).
Vasquez, F., Correa-Burrows, P., Blanco, E., Gahagan, S. & Burrows, R. A waist-to-height ratio of 0.54 is a good predictor of metabolic syndrome in 16-year-old male and female adolescents. Pediatr. Res. 85, 269–274 (2019).
Arnaiz, P. et al. Razón cintura estatura como predictor de riesgo cardiometabólico en niños y adolescentes. Rev. Chil. Cardiol. 29, 281–288 (2010).
Elizondo-Montemayor, L., Serrano-González, M., Ugalde-Casas, P. A., Bustamante-Careaga, H. & Cuello-García, C. Waist-to-height: cutoff matters in predicting metabolic syndrome in Mexican children. Metab. Syndr. Relat. Disord. 9, 183–190 (2011).
Rodea-Montero, E. R., Evia-Viscarra, M. L. & Apolinar-Jiménez, E. Waist-to-height ratio is a better anthropometric index than waist circumference and BMI in predicting metabolic syndrome among obese Mexican adolescents. Int. J. Endocrinol. 2014, 195407 (2014).
Santoro, N. et al. Predicting metabolic syndrome in obese children and adolescents: look, measure and ask. Obes. Facts 6, 48–56 (2013).
Kalavainen, M., Utriainen, P., Vanninen, E., Korppi, M. & Nuutinen, O. Impact of childhood obesity treatment on body composition and metabolic profile. World J. Pediatr. 8, 31–37 (2012).
Yli-Piipari, S. et al. A twelve-week lifestyle program to improve cardiometabolic, behavioral, and psychological health in Hispanic children and adolescents. J. Altern. Complement. Med. 24, 132–138 (2018).
Ranucci, C. et al. Effects of an intensive lifestyle intervention to treat overweight/obese children and adolescents. Biomed. Res. Int. 2017, 8573725 (2017).
Harder-Lauridsen, N. et al. A randomized controlled trial on a multicomponent intervention for overweight school-aged children – Copenhagen, Denmark. BMC Pediatr. 14, 273 (2014).
World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310, 2191–2194 (2013).
Funding
The project OBEMAT2.0 clinical trial received funding from the Ministry of Economy and Competitiveness of the Spanish Government “Acción Estratégica en Salud 2013–2016”, reference PI15/00970 and PI15/01411, co-funded by the European Union through funds for regional development (FEDER). The funding source had no role in the design of this study and will have no role during its execution, analyses, interpretation of the data, or in any decision to submit results. V.L. is the recipient of a Serra Hunter Fellowship.
Author information
Authors and Affiliations
Consortia
Contributions
J.M.-H. performed the analyses and drafted the article; V.L. and J.M.-H. conceived and designed the analyses; N.F., J.B., R.C.-M., and J.E. contributed with data and analysis tools. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study followed the rules of the Declaration of Helsinki.56 The ethical committees holding the activity of all the involved study centers approved the protocol. If any amendments to the protocol were made, the Ethics Committees were notified as necessary. All parents or legal guardians signed informed consent prior to study enrollment. Children aged 12 years or above signed informed assent to participate in the study as well.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muñoz-Hernando, J., Luque, V., Ferré, N. et al. Diagnosis accuracy of waist-to-height ratio to predict cardiometabolic risk in children with obesity. Pediatr Res 93, 1294–1301 (2023). https://doi.org/10.1038/s41390-022-02223-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02223-4