Abstract
Background
Urinary renin is proposed to be a novel prognostic biomarker of acute kidney injury (AKI) in adults. The intention of our study was to evaluate the early predictive value of urinary renin for AKI and pediatric intensive care unit (PICU) mortality in critically ill children.
Methods
The first available urine sample during the first 24 h after admission was collected upon PICU admission for the measurement of renin using ELISA. Urinary renin concentrations were corrected for urinary creatinine (urinary renin-to-creatinine ratio, uRenCR). AKI was defined based on KDIGO criteria.
Results
Of the 207 children, 22 developed AKI, including 6 with stage 1, 6 with stage 2, and 10 with stage 3, and 14 died during PICU stay. There was a significant difference in uRenCR between non-AKI children and those with AKI stage 3 (P = 0.001), but not with AKI stage 1 or 2. The uRenCR remained associated with AKI stage 3 and PICU mortality after adjustment for potential confounders. The area under the receiver operating characteristic curve of uRenCR for discrimination of AKI stage 3 was 0.805, and PICU mortality was 0.801.
Conclusions
Urinary renin was associated with the increased risk for AKI stage 3 and PICU mortality in critically ill children.
Impact
-
Urinary renin is proposed to be a novel prognostic biomarker of AKI in adult patients.
-
There are some differences between children and adults in physiological and pathophysiological characteristics.
-
This study demonstrated that urinary renin was associated with the increased risk for AKI stage 3 and PICU mortality in critically ill children.
-
Accurate identification of patients with severe renal injury or at high risk for mortality early in the disease course could augment the efficacy of available interventions and improve patient outcomes.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI), which is a common complication of critically ill patients, is recognized as an independent risk factor for mortality.1 A patient’s risk of both short-term and long-term adverse outcomes is correlated with the severity of AKI.2 Accurate identification of patients with severe renal injury or at high risk for mortality early in the disease course could augment the efficacy of available interventions and improve patient outcomes.
In current clinical practice, the diagnosis and staging of AKI still largely rely on serum creatinine (SCr) and urine output, which are known as insensitive and nonspecific indicators during acute changes in kidney function.3 In recent years, many biomarkers that may be useful for the detection of AKI before an increase in SCr have been proposed.4,5,6 However, the prognostic value of these biomarkers to predict adverse outcomes is limited.7,8 Up to now, there has been no consistent biomarker for the prediction of mortality in a general population of critically ill patients. Therefore, prognostic biomarkers that predict outcomes in patients are needed.
Renin is an aspartyl1 protease that catalyzes the first step in the activation of the renin–angiotensin–aldosterone system (RAAS).9 The amount of renin in the circulating and tissue determines the overall activity of the RAAS.10 Alge et al. identified urinary renin and angiotensinogen as novel prognostic biomarkers of AKI in adult patients.11 There are some differences between children and adults in physiological and pathophysiological characteristics. Previous studies suggest that the RAAS is influenced by age.12,13 Increased renin synthesis, immaturity of the feedback system, and diminished renin clearance from the circulation because of immature liver and kidneys, may contribute to high renin levels during infancy.12 Moreover, kidneys of children differ from adults in many aspects, such as a lack of maturation of glomerular filtration and tubular function, decreased renal perfusion pressure.14 For the above reasons, it is irrational to extrapolate adult data to children. However, little is known about the prognostic predictive power of urinary renin in critically ill children.
The aim of this study was to evaluate the early predictive value of urinary renin during the first 24 h after pediatric intensive care unit (PICU) admission for AKI and PICU mortality in critically ill children.
Methods
Study population
This observational prospective cohort study was conducted in the PICU at Children’s Hospital of Soochow University. Children aged 1 month to 16 years were recruited from PICU between December 2017 and January 2018. Exclusion criteria included age <1 month or over 16 years and failure to collect a urine sample within the first 24 h of PICU stay. For children who were admitted to PICU multiple times within a single hospital stay, only the last admission was included in the study. Patients were followed until the time of death or PICU discharge, whichever occurred first. The study was approved by the Institutional Review Board of the Children’s Hospital of Soochow University, and parental written informed consent was obtained for all participants.
Clinical data collection
Baseline patient characteristics were recorded within the first 24 h after PICU admission, including patients’ demographics, medical history, and the main reason for admission. The patient was evaluated regarding the need for mechanical ventilation (MV), and comorbidities including sepsis, shock, disseminated intravascular coagulation (DIC), and multiple organ dysfunction syndrome (MODS) were determined daily during PICU stay.
Urine sample collection and biomarker measurement
The first available urine sample during the first 24 h after admission was collected upon PICU admission for the measurement of renin and creatinine (Cr). Urine samples were aliquoted and stored at −80 °C within 1 h of collection until use. Each urine sample was centrifuged at 1500 × g at 4 °C for 15 min, and the supernatant was utilized to measure the urinary renin level by a human total renin enzyme-linked immunosorbent assay (ELISA) kit (Human Renin Quantikine ELISA DREN00, R&D Systems). We performed the assay according to the manufacturer’s protocol. The assay range was 31.3–2000 pg/mL, and the sample was diluted tenfold in reagent diluent to ensure that the enzymatic reaction was maintained within the assay range, if the testing material content was excessively high. The coefficients of variation of intra-assay and inter-assay were <10%, respectively. The levels of urinary renin were detectable in all samples. Urinary renin concentrations were corrected for urinary Cr (urinary renin-to-creatinine ratio, uRenCR) to eliminate the influence of different urinary flow rates on the results. The uRenCR from sample collecting within the first 24 h after PICU admission was considered as early excretion of urinary renin. The levels of urinary Cr were measured using the sarcosine oxidase method on an automatic biochemical analyzer (Hitachi 7600, Tokyo, Japan).
Assessment of illness severity
The severity of illness was determined using the pediatric risk of mortality III (PRISM III) score.15 Age-related physiological parameters, including systolic pressure, temperature, mental status, heart rate, pupillary reflexes, PH, partial pressure of carbon dioxide, total carbon dioxide, arterial partial pressure of oxygen, glucose, potassium, SCr, blood urea nitrogen, white blood cell count, prothrombin or partial thromboplastin time, and platelet count, were collected in the first 24 h after PICU admission.
Definition of AKI and clinical outcomes
The diagnosis and staging of AKI were determined based on SCr and urine output using the criteria in Kidney Disease: Improving Global Outcomes (KDIGO).2 Blood samples for SCr were collected and measured daily during the first 7 days of PICU stay, followed by routine measurement every 3–5 days until death or discharge from the PICU. Baseline SCr was defined as the lowest SCr in the 3 months prior to PICU admission. If the value was not available, the SCr at the time of PICU admission was used. For patients with elevated SCr ≥ 106.1 μmol/L at PICU admission, the lowest SCr value within 2 weeks during PICU stay was considered as the baseline Cr adapted from previous studies.16,17,18
The clinical outcome of interest was PICU mortality, which was defined as a death occurring during PICU stay after admission, including the death due to the withdrawal of treatment.
Statistical analysis
Statistical analyses were performed with the software of SPSS and Medcalc. We first checked the assumptions of normality and homogeneity of variance. Since all continuous variables were non-normally distributed, the continuous variables were presented as median with interquartile range and were compared using the Mann–Whitney U test or the Kruskal–Wallis H test. Categorical variables were compared using the chi-square test or Fisher’s exact test. Univariate and stepwise multivariate linear regression analyses were used to identify possible variables that may be associated with uRenCR. Continuous variables with skewed distribution were log10 transformed for linear regression analysis, and multicollinearity among variables was assessed by variance inflation factor (VIF) and tolerance values. Since kidney function is immature in children <2 years old14 and the renin activity is influenced by age,12 the patients were grouped into two age categories with 2 years of age as the cutoff value, and the Spearman’s analysis was used to analyze the correlation between uRenCR and age in different age groups. Univariate and multivariate logistic regression analyses were used to calculate the odds ratio (OR) and adjusted OR to assess the independent association of variables with AKI stage 3 and PICU mortality. Receiver operating characteristic (ROC) curves were constructed to assess the discriminative power of uRenCR and admission SCr for AKI, and the discriminative power of the laboratory variables with P < 0.05 in multivariate logistic regression analysis and the PRISM III score for AKI stage 3 or PICU mortality. The area under the ROC curve (AUC) was determined, and the nonparametric method of Delong was used to compare differences between AUCs. Optimal cutoffs were determined by selecting the data point that maximize both sensitivity and specificity on the ROC curve. P value < 0.05 (two-tailed) was considered statistically significant.
Results
Clinical diagnosis
The study enrolled 207 critically ill children. Of the total 226 children admitted to the PICU during the study period, 19 were excluded: 1 was at age under 1 month, 2 stayed in PICU for <24 h (discharge from the PICU or death before collecting urine sample), 4 had multiple PICU admissions within a single hospital stay, only the last admission was included in the analysis, and 12 had a failure in collecting urine samples during the first 24 h after PICU admission. Major admission diagnoses included respiratory diseases (41.5%), neurological diseases (17.4%), gastrointestinal disease (12.1%), hematologic diseases (9.2%), and sepsis (7.2%), and others (12.6%).
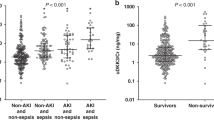
Of the 207 children, 22 (10.6%) developed AKI during PICU stay, including 6 with stage 1, 6 with stage 2, and 10 with AKI stage 3. Among AKI patients, 14 (63.6%) developed AKI on the first day of PICU admission. The PICU mortality rate of the whole cohort with and without AKI was 6.8% (95% CI: 3.3–10.2%). A comparison of the clinical characteristics among children with non-AKI, AKI stage 1, AKI stage 2, and AKI stage 3 is displayed in Table 1. The values of renin from the urine samples collected in the first 24 h after PICU admission in children were analyzed. There was a significant difference in the uRenCR among children in non-AKI, AKI stage 1, AKI stage 2, and AKI stage 3 groups (0.3 [0.1–0.6] vs. 0.8 [0.1–3.7] vs. 0.6 [0.3–1.0] vs. 1.4 [0.7–22.7] ng/mg, P = 0.004). Multiple comparisons of two groups were conducted after comparison among the four groups. Although there was no significant difference in uRenCR between non-AKI and AKI stage 1 groups (P = 0.378), and between non-AKI and AKI stage 2 groups (P = 0.128), the uRenCR was significantly higher in children with AKI stage 3 than that in those without AKI (P = 0.001). The comparison of uRenCR among different AKI status is shown in Supplemental Fig. S1 (online).
Correlation of uRenCR with clinical and laboratory variables
To identify possible variables that may be associated with uRenCR, clinical and laboratory variables in Table 1 were analyzed using univariate and multivariate linear regression analyses. Variables potentially associated with uRenCR in the univariate linear regression analysis are displayed in Table 2. To identify whether these variables were independently associated with uRenCR, the stepwise multivariate linear analysis was further conducted. The association of uRenCR with body weight (P < 0.001), PRISM III score (P < 0.001), duration of MV (P = 0.004), serum sodium (P = 0.001), serum chlorine (P = 0.003), and serum glucose (P = 0.001) remained significant in the final model. The children with AKI remained associated with higher uRenCR in multivariate analysis (P < 0.001). VIF and tolerance values confirmed the absence of significant multicollinearity between variables in the final model of multivariate regression analysis.
Since children differ from adults in many aspects, especially in children younger than 2 years of age, the patients were grouped into two age categories: ≤2 years (n = 129) and >2 years (n = 78), according to the stages of growth which was described in the original study.14 The correlation between uRenCR and age remained significant in ≤2 years group (r = −0.181, P = 0.04), but not in >2 years group (r = −0.163, P = 0.154).
Association of uRenCR with AKI and AKI stage 3
Univariate and multivariate logistic regression analyses were used to analyze the association between uRenCR and AKI. The uRenCR was associated with AKI (OR = 1.026, 95% CI: 1.001–1.052, P = 0.045) in the univariate logistic regression analysis. However, the association did not remain significant after adjustment for body weight, sex, and PRISM III score (OR = 1.017, 95% CI: 0.988–1.047, P = 0.254) in the multivariate logistic regression analysis.
The admission SCr displayed an AUC of 0.783 (P < 0.001) for discrimination of AKI, which was higher than uRenCR (AUC = 0.720, P = 0.001). However, the difference between the two AUCs of admission SCr and uRenCR did not reach statistically significant in discriminating AKI children from non-AKI children (AUC = 0.783 vs. AUC = 0.720, P = 0.529). The admission SCr displayed a sensitivity of 68.2% and a specificity of 89.2% at the optimal cutoff value of 42.95 μmol/L. The uRenCR displayed a sensitivity of 59.1% and a specificity of 85.4% at the optimal cutoff value of 0.91 ng/mg.
To identify whether uRenCR was independently associated with AKI stage 3 in children, all variables in Table 1, which were considered confounding factors, were used for logistic regression analyses in Table 3. In the multivariate logistic analysis, the uRenCR remained significantly associated with AKI stage 3 after adjustment for body weight and sex (P = 0.005).
The discriminative performance of uRenCR for AKI stage 3 is displayed in Table 4 and Supplemental Fig. S2 (online). The uRenCR achieved AUC of 0.805, which was similar to the results obtained based on admission SCr (AUC = 0.883) and PRISM III score (AUC = 0.821), followed by serum albumin (AUC = 0.780) and serum chlorine (AUC = 0.707), for discrimination of AKI stage 3. When combining uRenCR with admission SCr, PRISM III score, serum albumin, and serum chlorine, the discriminative performance improved (AUC = 0.897) over that uRenCR alone, but not reaching statistical significance (P = 0.196). At the optimal cutoff value of 0.91 ng/mg, the sensitivity and specificity were 80.0% and 83.8%, respectively, for uRenCR to discriminate AKI stage 3.
Association of uRenCR with PICU mortality
To identify whether uRenCR was independently associated with PICU mortality in critically ill children, all variables in Table 1, which were considered confounding factors, were used for logistic regression analyses in Table 5. In the multivariate logistic analysis, the association of uRenCR with mortality remained significant after adjustment for body weight, sex, and illness severity as assessed by PRISM III score (P = 0.006). The association of uRenCR with mortality also remained significant after adjustment for body weight, sex, PRISM III score, MV, duration of MV, AKI, sepsis, MODS, and shock/DIC (P = 0.020). The comparison of uRenCR between survivors and non-survivors is shown in Supplemental Fig. S3 (online). Non-survival children had significantly higher uRenCR compared to survivors (1.52 [0.52–22.75] vs. 0.25 [0.13–0.67] ng/mg, P < 0.001).
The predictive ability of uRenCR for PICU mortality is shown in Table 6 and Supplemental Fig. S4 (online). The uRenCR displayed the higher AUC of 0.801, followed by the PRISM III score (AUC = 0.797), for predicting PICU mortality in children. Combining uRenCR with PRISM III score improved the predictive performance (AUC = 0.849); which, however, was not significantly better than uRenCR alone (AUC = 0.849 vs. AUC = 0.801, P = 0.491) in predicting PICU mortality. At the optimal cutoff value of 0.80 ng/mg, the sensitivity and specificity were 71.4% and 81.3%, respectively, for uRenCR to predict PICU mortality.
Discussion
This study provides data on urinary renin concentrations in critically ill children and demonstrates that the urinary renin level upon admission to PICU is significantly associated with AKI stage 3 and mortality during PICU stay.
Renin, as a specific endopeptidase, converts angiotensinogen to angiotensin I, which governs the rate limiting step in the generation of RAAS metabolites.9 Previous study suggests urinary renin, but not angiotensinogen or aldosterone, reflects renal RAAS activity.19 The components of RAAS in urine have been described as novel prognostic biomarkers of AKI;11,20 however, rarely does a research focus on the prognostic value of urinary renin for AKI. In a multicenter, retrospective cohort study, elevated urinary renin at the time of diagnosis with AKI stage 1 was associated with AKI stage 3 or mortality in patients who had cardiac surgery, and addition of urinary angiotensinogen and urinary renin to a clinical model improved prediction of the outcome.11 In another study conducted in adult patients with AKI stage 1 after cardiac surgery, 32 biomarkers in the urine were measured, and urinary renin was shown to be a moderately strong predictor for AKI stage 3 or mortality.21 Both of the two studies were focused specifically on AKI caused by cardiac surgery. However, AKI can be caused by many factors, and it is common among critically ill patients.22 In our study, we focused on children admitted to PICU with diverse etiologies. Our results demonstrated that urinary renin was significantly associated with AKI stage 3, and it may act as an early biomarker for discrimination of AKI stage 3 in critically ill children.
The discriminative ability of urinary renin for children in our study is larger than the results in the previous studies of adults.11,21 We speculate that this may be caused by the differences of physiological and pathophysiological characteristics between children and adults. In addition, the different time of collecting urine samples may also affect the discriminative ability. In our study, urine samples were collected and used for analysis as early as possible, during the first 24 h after PICU admission, regardless of whether the diagnostic criteria for AKI had been met.
The goal of most AKI biomarker research is early identification of AKI, and multiple biomarkers have been proposed, such as neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), fatty-acid-binding protein-1 (FABP-1), tissue inhibitor of metalloproteinase-2 (TIMP-2), and insulin-like growth factor-binding protein-7 (IGFBP-7).23,24,25,26,27 Of these studies, a number of focused on critically ill children with different causes of AKI. Urinary NGAL had an AUC of 0.78 for AKI and an AUC of 0.61 for severe AKI defined as injury and failure of the pediatric modified risk, injury, failure, loss, end stage kidney disease (pRIFLE) criteria.25 Urinary IL-18 and L-FABP measurements on the day of PICU admission had AUCs of 0.82 and 0.59, respectively, for AKI prediction.26 Urinary [TIMP-2]•[IGFBP-7] had an AUC of 0.74 for AKI and an AUC of 0.75 for severe AKI.27 Compared with previous studies, our results demonstrated urinary renin had a superior discrimination for AKI stage 3 (AUC = 0.805), implying that urinary renin is promising as a novel prognostic biomarker of AKI stage 3 in critically ill children.
A negative correlation was found between urinary renin levels and age in our study. However, the correlation was not significant in critically ill children aged >2 years. It has been showed that plasma renin activity is inversely correlated with age in healthy children,28 and the high plasma renin activity and concentrations in neonates decline steeply during infancy, and subsequently decrease to adult levels gradually.12 Our results demonstrate an inverse relationship between urinary renin and age in children, but the relationship may disappear after 2 years of age.
In our study, there was an inverse correlation of urinary renin levels with serum sodium, and a positive correlation with serum glucose levels. The relationship between RAAS activation and sodium or glucose has been demonstrated by in vivo and in vitro studies.29,30,31,32 In murine kidney, the cells of connecting segment and collecting duct released renin to tubular lumen in response to salt depletion.29 In human, low sodium intake significantly increased plasma renin concentration compared with high sodium intake.30 In addition to being related to sodium, the activation of RAAS is also associated with high glucose exposure. Studies showed that high glucose treatment enhanced the renin expression in cultured collecting duct cells,31 and triggered renin synthesis and release in animal kidneys.32 Our data confirm these findings and provide the information with regard to the relationship of urinary renin, with serum sodium and serum glucose in human.
Another important result of this study was the positive correlation of urinary renin with the PRISM III score, which is a valid measure of illness severity in the first 24 h after admission in the pediatric population.15,33,34 This raises the question of whether urinary renin levels are associated with clinical outcomes in critically ill children. Our data indicate that urinary renin is an independent variable associated with mortality, even after adjusting for potential confounders, including the severity of illness assessed by the PRISM III score.
There are few studies on the predictive value of urinary renin on mortality in critically ill patients. Our study demonstrates that the prognostic accuracy of urinary renin and the PRISM III score obtained within the first 24 h after PICU admission are comparable in predicting mortality, which suggests that urinary renin at admission may be used to assess mortality risk or as an auxiliary indication for it is simple to use. Of course, a multicenter study is needed, and a larger quantity of samples should be investigated to further demonstrate the results.
The present study has some limitations. First, it is a single-center study of limited size, which may lead to a difference in the incidence of AKI compared with other studies, and the slight imbalance between the incidence of AKI and mortality may limit the generalizability of our results. Besides, the low number of children with AKI stage 3 and deaths may lead to a type 1 error. Second, since AKI might be underestimated by using admission SCr as baseline SCr, the lowest SCr value within 2 weeks during PICU stay was used as the baseline SCr for patients with elevated SCr (≥106.1 μmol/L) at PICU admission, which, however, might overestimate the incidence of AKI. Nevertheless, previous study demonstrated that, a measured rather than estimated value should be used for baseline Cr.16 Third, although our study is consistent with a previous study, indicating that the majority of children developed AKI within the first 24 h of admission to the PICU,35 AKI occurred in the first week of PICU admission, with 63.6% in the first day, implying that critically ill children might be admitted later to the PICU, which may limit the generalizability of our results to critically ill children admitted earlier in their critical illness courses in the PICU. Moreover, although the original intention of our study was to evaluate the predictive value of urinary renin for AKI, but for the above reasons, stating that the urinary renin may predict AKI might be a gross overstatement. Fourth, the discriminative ability of urinary renin for AKI stage 3 or mortality was similar to the results obtained based on admission SCr or the PRISM III score. However, since 14 (63.6%) patients developed AKI on the first day of PICU admission, and the sample size of patients who developed AKI after the first day of PICU admission was small, we cannot explore whether the discriminative value of urinary renin for AKI or AKI stage 3 is better than admission SCr if elevated at time. In the other hand, the association of urinary renin, but not admission SCr, with PICU mortality remained significant after adjustment for illness severity, suggesting that the clinical significance of urinary renin might be over SCr at admission for prediction of mortality. Fifth, to evaluate the early predictive value of urinary renin, we used the urine samples collected as early as possible during the first 24 h after PICU admission for analysis rather than serial measurements, which might affect the predictive power of urinary renin. Sixth, urinary renin levels were measured by using a Human Renin Quantikine ELISA kit in the study, raising the question of the specificity of the kit.36 Nevertheless, this previous study demonstrates that total renin measurements by ELISA, immunoradiometric assays, and enzyme kinetic assay are highly correlated, suggesting that the predictive value of urinary renin may not be affected by the measurement method.36
Conclusions
Urinary renin is significantly associated with AKI stage 3 and PICU mortality in critically ill children even after adjustment for confounders. Urinary renin on admission to the PICU may be an early biomarker for discrimination of AKI stage 3 and PICU mortality in critically ill children. Future multicenter prospective studies are warranted to confirm the effectiveness of using urinary renin to provide prognostic information in critically ill children.
References
James, M. T., Bhatt, M., Pannu, N. & Tonelli, M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat. Rev. Nephrol. 16, 193–205 (2020).
Palevsky, P. M. et al. KDOQI US commentary on the 2012 KDIGO Clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 61, 649–672 (2013).
Murray, P. T. et al. A framework and key research questions in AKI diagnosis and staging in different environments. Clin. J. Am. Soc. Nephrol. 3, 864–868 (2008).
Zappitelli, M. et al. Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr. 169, 583–591 (2015).
Hanna, M. et al. Early urinary biomarkers of acute kidney injury in preterm infants. Pediatr. Res. 80, 218–223 (2016).
Chen, J. et al. The effectiveness of urinary TIMP-2 and IGFBP-7 in predicting acute kidney injury in critically ill neonates. Pediatr. Res. 87, 1052–1059 (2020).
Parr, S. K. et al. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. 87, 640–648 (2015).
Malhotra, R. & Siew, E. D. Biomarkers for the early detection and prognosis of acute kidney injury. Clin. J. Am. Soc. Nephrol. 12, 149–173 (2017).
Wu, C. H. et al. Renin-angiotensin system and cardiovascular functions. Arterioscler. Thromb. Vasc. Biol. 38, e108–e116 (2018).
Sparks, M. A., Crowley, S. D., Gurley, S. B., Mirotsou, M. & Coffman, T. M. Classical renin-angiotensin system in kidney physiology. Compr. Physiol. 4, 1201–1228 (2014).
Alge, J. L. et al. Association of elevated urinary concentration of renin-angiotensin system components and severe AKI. Clin. J. Am. Soc. Nephrol. 8, 2043–2052 (2013).
Bauer, J. H. Age-related changes in the renin-aldosterone system physiological effects and clinical implications. Drugs Aging 3, 238–245 (1993).
Genovesi, S. et al. Aldosterone-to-renin ratio depends on age and sex in children attending a clinic for cardiovascular risk assessment. J. Hypertens. 36, 344–352 (2018).
Rodieux, F., Wilbaux, M., van den Anker, J. N. & Pfister, M. Effect of kidney function on drug kinetics and dosing in neonates, infants, and children. Clin. Pharmacokinet. 54, 1183–1204 (2015).
Pollack, M. M., Patel, K. M. & Ruttimann, U. E. PRISM III: an updated pediatric risk of mortality score. Crit. Care Med. 24, 743–752 (1996).
Pickering, J. W. & Endre, Z. H. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin. J. Am. Soc. Nephrol. 5, 1165–1173 (2010).
Li, Y. et al. Early fluid overload is associated with acute kidney injury and PICU mortality in critically ill children. Eur. J. Pediatr. 175, 39–48 (2016).
Fang, F. et al. Subclinical acute kidney injury is associated with adverse outcomes in critically ill neonates and children. Crit. Care 22, 256 (2018).
van den Heuvel, M. et al. Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin-angiotensin-aldosterone system activity and the efficacy of renin-angiotensin-aldosterone system blockade in the kidney. J. Hypertens. 29, 2147–2155 (2011).
Alge, J. L. et al. Urinary angiotensinogen and risk of severe AKI. Clin. J. Am. Soc. Nephrol. 8, 184–193 (2013).
Arthur, J. M. et al. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 85, 431–438 (2014).
Hoste, E. A. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
Krawczeski, C. D. et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J. Am. Coll. Cardiol. 18, 2301–2309 (2011).
Bihorac, A. et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am. J. Respir. Crit. Care Med. 189, 932–939 (2014).
Zappitelli, M. et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit. Care 11, R84 (2007).
Dart, A. B. et al. Biomarkers for early acute kidney injury diagnosis and severity prediction: a pilot multicenter canadian study of children admitted to the ICU. Pediatr. Crit. Care Med. 18, 239–244 (2017).
Westhoff, J. H. et al. Urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) • insulin-like growth factor-binding protein 7 (IGFBP7) predicts adverse outcome in pediatric acute kidney injury. PLoS ONE 10, 143628 (2015).
Martinez-Aguayo, A. et al. Aldosterone, plasma Renin activity, and aldosterone/renin ratio in a normotensive healthy pediatric population. Hypertension 56, 391–396 (2010).
Rohrwasser, A. et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 34, 1265–1274 (1999).
Graudal, N. A., Hubeck-Graudal, T. & Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst. Rev. 4, CD004022 (2017).
Leite, A. P. O. et al. Modulation of renin angiotensin system components by high glucose levels in the culture of collecting duct cells. J. Cell Physiol. 234, 22809–22818 (2019).
Toma, I. et al. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J. Clin. Investig. 118, 2526–2534 (2008).
Brady, A. R. et al. Assessment and optimization of mortality prediction tools for admissions to pediatric intensive care in the United kingdom. Pediatrics 117, e733–e742 (2006).
Gemke, R. J. & van Vught, J. Scoring systems in pediatric intensive care: PRISM III versus PIM. Intensive Care Med. 28, 204–207 (2002).
Wheeler, D. S. et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit. Care Med. 36, 1297–1303 (2008).
Roksnoer, L. C. et al. Methodologic issues in the measurement of urinary renin. Clin. J. Am. Soc. Nephrol. 9, 1163–1167 (2014).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81971432 and 81370773); Jiangsu Province Science and Technology Support Program (BE2020660); Natural science foundation of JiangSu province (BK20171217); and Key talent of women’s and children’s health of JiangSu province (FRC201738). The funders had no role in study design, data collection, preparation of the manuscript, and decision to publish.
Author information
Authors and Affiliations
Contributions
Y.K. participated in data analysis and drafted the manuscript. H.H. performed the experiments. X.D., Z.Z., Z.B., and J.C. participated in collecting the data and samples. F.F., J.P., and X.L. participated in data analysis and interpretation. J.W. participated in the design of the study and coordination. Y.L. had primary responsibility for study design, performing the experiments, data analysis, interpretation of data, and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent statement
The study was approved by the Institutional Review Board of the Children’s Hospital of Soochow University, and parental written informed consent was obtained for all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuai, Y., Huang, H., Dai, X. et al. In PICU acute kidney injury stage 3 or mortality is associated with early excretion of urinary renin. Pediatr Res 91, 1149–1155 (2022). https://doi.org/10.1038/s41390-021-01592-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01592-6
This article is cited by
-
Derivation and validation of urinary TIMP-1 for the prediction of acute kidney injury and mortality in critically ill children
Journal of Translational Medicine (2022)
-
Comparison of diagnostic criteria for acute kidney injury in critically ill children: a multicenter cohort study
Critical Care (2022)