Abstract
Background
Three-dimensional printing (3DP) addresses distinct clinical challenges in pediatric care including: congenital variants, compact anatomy, high procedural risk, and growth over time. We hypothesized that patient-specific applications of 3DP in pediatrics could be categorized into concise, discrete categories of use.
Methods
Terms related to “three-dimensional printing” and “pediatrics” were searched on PubMed, Scopus, Ovid MEDLINE, Cochrane CENTRAL, and Web of Science. Initial search yielded 2122 unique articles; 139 articles characterizing 508 patients met full inclusion criteria.
Results
Four categories of patient-specific 3DP applications were identified: Teaching of families and medical staff (9.3%); Developing intervention strategies (33.9%); Procedural applications, including subtypes: contour models, guides, splints, and implants (43.0%); and Material manufacturing of shaping devices or prosthetics (14.0%). Procedural comparative studies found 3DP devices to be equivalent or better than conventional methods, with less operating time and fewer complications.
Conclusion
Patient-specific applications of Three-Dimensional Printing in Medicine can be elegantly classified into four major categories: Teaching, Developing, Procedures, and Materials, sharing the same TDPM acronym. Understanding this schema is important because it promotes further innovation and increased implementation of these devices to improve pediatric care.
Impact
-
This article classifies the pediatric applications of patient-specific three-dimensional printing.
-
This is a first comprehensive review of patient-specific three-dimensional printing in both pediatric medical and surgical disciplines, incorporating previously described classification schema to create one unifying paradigm.
-
Understanding these applications is important since three-dimensional printing addresses challenges that are uniquely pediatric including compact anatomy, unique congenital variants, greater procedural risk, and growth over time.
-
We identified four classifications of patient-specific use: teaching, developing, procedural, and material uses.
-
By classifying these applications, this review promotes understanding and incorporation of this expanding technology to improve the pediatric care.
Similar content being viewed by others
Introduction
Pediatrics makes demands unlike any other medical specialty: clinicians must tackle compact anatomy, often with unique congenital features, and make decisions that benefit children not only in the moment but also as they grow over time. These demands require a personalized medicine approach to provide optimal care to young patients. The emerging technology of three-dimensional printing (3DP) enables this level of individualization by printing the patient’s unique anatomy for patient-specific models, with applications that range from patient education to surgical intraoperative use.1 Clinical 3DP items most commonly employ additive manufacturing, in which a digitized 3D model is manipulated and then printed in successive layers to build the desired object.1,2,3,4 The term “additive manufacturing” is a broad category, covering more nuanced processes such as stereolithography, selective laser sintering, and fused deposition modeling which can process a wide array of materials such as metals, polymers, or even ceramics into the desired shape.5 The result is the rapid production of high-fidelity, personalized models that can be used in complex care management.
The current literature suggests 3DP is a powerful tool that enhances clinical care; however, previous reviews were largely limited to adult care in single surgical subspecialties.2,3,6,7 We propose that 3DP has the potential to be exceptionally valuable in the pediatric setting, as it presents elegant solutions to the challenges of caring for young patients. Tangible 3DP objects provide pediatric clinicians distinct advantages, including: facilitating communication with worried parents, assisting providers in clinical decision-making, allowing physicians to visualize complex congenital defects, and enhancing precision in complicated procedures. By analyzing and classifying patient-specific 3DP items in this new comprehensive taxonomy, we promote increased understanding and implementation of these powerful instruments of personalized medicine across the many fields of medical and surgical specialties.
This is the first systematic review of patient-specific 3DP medical applications that distinctly focuses on pediatric patients across all specialties. In this study, we categorize the way pediatric patient-specific 3DP devices are employed, compare 3DP devices against conventional methods, and identify opportunities for future integration of 3DP in the care of pediatric patients.
Methods
Inclusion and exclusion criteria
This systematic review focuses on the pediatric applications of patient-specific three-dimensional printing (3DP) in pediatric patient-specific care. 3DP objects in this study were designed through additive manufacturing—the deposition of material in precise, successive layers to build the desired shape. Inclusion criteria required that studies: (1) manufacture 3DP objects through additive manufacturing, (2) involve the use of a patient-specific 3DP object in the clinical care of a patient either directly or indirectly, (3) report primary data, and (4) address the pediatric population, defined as patients up to and including the age of 18 years old. Studies analyzing qualitative data from adults about pediatric patients (e.g., a parent’s understanding of his or her child’s condition) were included as long as the patients themselves were pediatric. Both case studies and case series were included. All available publication years were considered.
Exclusion criteria were as follows: (1) reviews, technique articles, editorials, book chapters, methods papers, and incomplete articles (e.g., only abstracts available), (2) articles not available in English, (3) articles in which the 3DP object was not directly or indirectly involved in some aspect of patient care, (4) objects produced by methods other than additive manufacturing, including subtractive manufacturing, computer numerical control milling, and prototype machining, (5) studies that included patients ages 19 and older, (6) 3DP objects that were not patient-specific, meaning the same object was used in the care of multiple patients, (7) cadaver studies, (8) animal studies, and (9) tissue engineering proof-of-concept studies or tissue engineering studies conducted solely in vitro.
Systematic database search
The medical literature published in five databases (Pubmed MEDLINE, Scopus, Ovid, COCHRANE Central, and Web of Science) was searched for articles that included terms relating to “three-dimensional printing” and “pediatrics.” The Medical Subject Heading terms “printing, three-dimensional,” “pediatrics,” “pediatric surgery,” “adolescent medicine,” and “infant” were included, as well as wildcard asterisked terms, to systematically review available literature.
Search results from all databases were combined, and duplicates were removed. Two reviewers independently conducted title weeds and abstract weeds for concordance of article relevance. The full-text of the relevant studies was examined for eligibility, and disagreements were discussed to reach consensus.
Data collection
Data collected from the individual articles included manufacturing variables, specialty usage, comparison data and patient-specific applications. Manufacturing data included medical imaging used to obtain data, modeling software, printers, time, and cost. Specialty was defined as the medical specialty of the senior author. Patient-specific applications were classified into broad categories of application. If the application was surgical, the type of operation was noted. Lastly, studies were searched for any comparison data that could suggest use of 3DP objects equal or superior to conventional methods.
Results
The multiple database search was completed on January 11, 2018, with results combined and duplicates removed. The search delivered 2122 unique articles, and a title weed eliminated 1303 articles based on inclusion and exclusion criteria. An abstract weed was conducted on the 819 remaining articles (Cohen’s kappa k = 0.968), and a full-text weed reviewed the resulting 367 articles (k = 0.949). In total, 139 articles met all criteria and were eligible for inclusion in systematic review. The attrition flowchart detailing study selection is shown in Fig. 1.
These papers contained a total of 508 patients, with an average of 3.6 patients included per study. Mean patient age was 7.6 years old. Of the 139 full-text articles reviewed, 8 were Level II, 8 were Level III, 37 were Level IV, and 86 were Level V evidence, using guidelines for therapeutic studies.8 Manufacturing variables, specialty usage, comparison of 3DP vs. conventional methods, and patient-specific clinical applications were all examined and synthesized into a new classification schema of three-dimensional printing (3DP) use.
Manufacturing variables
Three-dimensional printing begins with the acquisition of patient data. Most commonly, these data were generated through computed tomography (CT) imaging (62.8% of all patients), but a fraction of patients did not have any medical imaging (3.4%) and instead had direct measurements (e.g., hand measurements of the contralateral side for hand prosthetics). Next, patient data were converted into a 3D digital rendering. These models could be either positive-space models mimicking actual patient anatomy or negative-space models representing the space surrounding the patient anatomy. Modeling could also be used to manipulate images into virtual positions (i.e., existing digitally only). One commonly employed manipulation was mirroring, where an ipsilateral defect is modeled with the contralateral normal side virtually reversed, to provide the ideal template.9,10,11,12,13,14,15 Consequently, 3DP devices could either be positive or negative-space models of actual or virtual patient anatomy. Modeling data were then converted to printable data. A wide variety of software was used across studies, with over 25 unique software company platforms reported. Materialise (Leuven, Belgium) was the most common (19.7%). Studies also demonstrated diversity in printer selection, utilizing 20 unique printers with Stratasys (Eden Prairie, MN) as the most frequent (13.4%).
Additional factors compared across 3DP objects included time and cost. Seventy-four patients10,16,17,18,19,20,21,22,23,24,25,26,27,28,29 (14.6%) had information available regarding production time, ranging from 0.42 to 108 hours (average 14.4 hours). Sixty-nine patients16,26,30,31,32,33,34,35,36 (13.6%) had the cost of 3DP devices listed ranging from $20.75 to $4043 (average cost $895.80). Manufacturing variables are further outlined in Table 1.
Specialty use
By specialty analysis, Orthopedic Surgery had the highest volume of patients (28.8%), followed by Plastic Surgery (13.9%). When evaluating how these specialties employed 3DP items, many uses were interdisciplinary. For example, 3DP cutting guides for spinal surgery were employed by both orthopedic and neurosurgeons. To account for this interdisciplinary overlap, specialty use was also tabulated by anatomic area. Anatomic areas included: craniofacial (skull, face, jaws),9,10,11,12,13,14,15,23,31,32,33,35,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92 central nervous system (brain, spine),34,93,94,95,96,97,98,99,100 cardiothoracic (heart, lungs, chest),24,25,26,27,28,36,93,94,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121 upper extremity,16,17,19,21,22,29,30,122,123,124,125,126,127 lower extremity,128,129,130,131,132,133,134,135,136 gastrointestinal (abdomen and its viscera),18,137,138,139 and genitourinary,140,141,142 with distributions listed in Fig. 2. Craniofacial was the highest anatomic area that employed 3DP (37.1%), with the majority of contributions from the specialty of Plastic Surgery.
Comparison studies
Only a handful of articles were pediatric comparative studies that compared 3DP models with conventional methods (n = 6). These six comparison studies32,94,96,121,128,129 focused primarily on procedural applications. Findings generally indicated incorporation of 3DP devices to be equivalent to or better than conventional methods, with shorter operating times, shorter fluoroscopy exposure, more accurate hardware placement, and fewer complications.
Classification
We identified four major classifications of patient-specific 3DP applications in pediatric patients: 1. Teaching, 2. Developing, 3. Procedures, and 4. Materials, each with subtypes as listed in Fig. 3.
Class 1. Teaching classification (9.3% of all patients) most often conveyed a disease process or treatment plan to patients and their families27,33,34,101,102,121,134,140,142,143 (6.0% all patients), but were also employed in an inter-professional setting to communicate between healthcare professionals26,102,121 (3.3% of all patients). In one common example,27,101,102,121 3DP teaching models allowed parents to directly visualize their child’s congenital heart disease, facilitating discussion about their child’s condition. Beyond patient education, 3DP devices can be used to teach the entire clinical team; in one example of clinician education, patient-specific cardiac models were shared when transitioning care from surgeons to ICU nurses postoperatively, which empowered nurses to tailor patient care in each unique case.26 Cardiothoracic applications were most common, comprising 81.4% of all teaching applications.
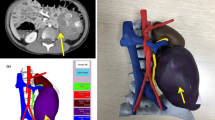
Class 2. Developing classification (33.8%) helped clinicians with two important functions: (1) determining the appropriateness of a specific diagnosis or intervention9,17,24,27,28,33,35,36,40,54,57,59,66,71,74,76,90,98,99,103,104,106,107,108,111,112,117,119,120,123,126,134,137,139,142,143,144,145 (termed Decision, 15.7% of all patients), or (2) practicing a given procedure on a patient-specific replica20,25,41,46,51,58,73,78,85,91,93,97,105,113,114,116,128,131,138,141 (termed Simulation, 18.1% of all patients), both for surgical operations and other procedures such as cardiac catheterizations. At times, decision models revealed that a procedure was unnecessary or unlikely to improve a patient’s condition. In one example, a 3DP model of a complex ventricular septal defect, initially imaged with echocardiogram and CT, allowed the clinician better visualization of the defect and driving the decision not to operate and sparing the child considerable morbidity.111 Simulation models also aimed to reduce morbidity by increasing precision of complex surgeries; for example, 3DP was used to accurately simulate a laparoscopic adrenalectomy for neuroblastoma complete with 3DP renderings of the tumor, the surrounding anatomy and even the outer abdominal cavity.141 Developing applications were used most commonly for central nervous system applications, particularly in planning for spinal surgery (30.3% of all patients).
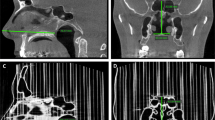
Class 3. Procedures classification refers to 3DP objects used intraoperatively to facilitate procedures (42.9%); it was further subdivided into four subtypes: contour model, guides, splints, and implants. Type I—Contour models10,11,13,14,20,29,32,34,46,48,52,53,60,61,62,64,67,68,72,82,84,86,89,96,109,110,118,122,125,135,136 (10.8%) are positive-space models based on either real or virtually modeled anatomy, such as a virtually mirrored contralateral ear, printed and used as a reference to reconstruct a congenitally absent ear.13 Type II—Guides18,29,30,37,43,44,45,70,77,80,81,94,95,122,128,129,132,136 (24.6%) are negative-space models based on actual patient anatomy, designed to contour to a segment of anatomy and allow for precise cutting or drilling that avoids critical structures, enabling precise screw placement in spinal surgery, for example.34,94,95 Type III—Splints31,65,73,75 (0.9%) are similar to guides in that they were negative-space 3DP items; however, unlike guides, splints were not based on preoperative anatomy but on virtual final simulated postoperative positions that were designed by virtual surgical planning. They are often used to hold anatomy in an interim position. In one example, a patient with coronal synostosis underwent reconstructive surgery to advance her frontal bone; the frontal bone was split in pieces, repositioned, and splints were used to hold the bone in ideal position before joining the fragments together with plates and screws.31 Finally, Type IV—Implants9,15,23,39,55,56,63,69,79,83,100,115,133,146,147,148 (6.6%) are either positive-space 3DP implantable materials or negative-space 3DP molds into which nonprintable materials are poured. Examples include thoracic vertebrae implants used to reconstruct the spine in a child with a primary bone tumor.147 Half of all operative uses (50%) were in craniofacial applications.
Class 4. Materials classification refers to external materials that can be removed or changed (14.0% of all patients), via Shaping12,38,42,47,49,88 (6.7%) and Substitution16,19,21,22,124,127,130 (7.3%). Shaping devices mold patient anatomy over time, taking advantage of pediatric growth to influence results. For children with orofacial clefts, presurgical 3DP devices narrowed the gap between alveolar segments and reshaped the nostril, potentially improving symmetry and potentially reducing later secondary revisions.38,42,47 Substitution items are external devices that replace normal anatomy (i.e., a prosthesis). 3DP prosthetic substitution objects can be rapidly printed at a relatively low cost that facilitates the frequent replacement in a growing child, allowing sequential substitution items to “grow” with a child, such as providing serial hand prosthetics that are size appropriate.16,19,21,22,124,127 In contrast to implants, these items are removable and do not require an operation to place or remove. These items were used most commonly in Craniofacial Surgery (50.7%), typically used as shaping devices in the care of children with orofacial clefts.
Comparison studies
Only a handful of articles were pediatric comparative studies that compared 3DP models with conventional methods (n = 6). These six comparison studies, summarized in Table 2, focused primarily on procedural applications. Findings generally indicated incorporation of 3DP devices to be equivalent to or better than conventional methods, with shorter operating times, less fluoroscopy exposure, more accurate hardware placement, and fewer complications.
Discussion
The body of literature on three-dimensional printing (3DP) is rapidly expanding: of the past decade of articles available on PubMed, one-third were published in the last year of this systematic review alone. Despite this growing knowledge, previous systematic reviews have usually been more focused on adult patients, and limited to subspecialties such as Plastic Surgery,1 Orthopedic Surgery,7 and Otolaryngology.6 This study uniquely includes medical and surgical applications in all specialties, focused on pediatric patients, a specialized population with unique problems that can benefit from this technology.
We were surprised to discover that with this broad range of patient-specific 3DP clinical applications, representing a wide array of medical specialties and anatomic regions, could be elegantly classified into four main classes: Class 1. Teaching to clarify the disease (for Patients and their families, or the medical Staff), Class 2. Developing a diagnosis or plan (Decision of diagnosis or intervention, or Simulation of procedure), Class 3. Procedures utilizing patient-specific 3DP models (Contour models to represent positive anatomy, Guides to avoid critical structures, Splints to set final virtual simulated positions, Implants to replace anatomy), and Class 4. Materials (Shaping devices to mold growing anatomy, or Substitution prosthetics to replace growing anatomy) (see Fig. 3). This useful classification scheme can be easily remembered, as the acronym for our taxonomy (TDPM: Teaching, Developing, Procedures, Materials) is the same as that for Three-Dimensional Printing in Medicine.
Consistent with previous reviews, we found that the majority of literature was generated by surgeons. This may be explained by 3DP’s ability to create high-fidelity anatomic models, which naturally benefits surgeons grappling to understand compact, complex, high-risk pediatric anatomy. In the procedures setting, 3DP was used most commonly in the care of patients with craniofacial deformities (skull, jaws, face). This is consistent with previous reviews of surgical literature, which describe Craniofacial Surgery as the most prevalent surgical application of 3DP.
Our proposed taxonomy broadly covers surgical and nonsurgical, incorporating the largest number of pediatric patients to date. Previous surgical reviews2 of 3DP devices subcategorized studies as anatomic models, surgical instruments, or prosthetics, a system that lacks the granularity of our classification. For example, it does not differentiate between surgical guides and splints, which have distinctly different computing requirements to generate virtual anatomy for the latter. Other medical reviews of 3DP devices149 have also highlighted some categories included in our system such as preoperative planning and patient−doctor communication, but did not account for the complexity of intraoperative uses detailed in this study. Furthermore, our study incorporates previously existing work: Our Class 3 Procedures found the same subtyping of contour, guides, splints, and implants, consistent with what was first described by Jacobs and Lin1 in their review of craniomaxillofacial surgery, showing that their categorization remains robust for our pediatric-only systematic review. Therefore, our new taxonomy integrates previous studies and builds upon them to create a comprehensive characterization of 3DP across the field of pediatric medicine.
Although over two-thirds of patients had surgical applications of 3DP, this study highlights the breadth of 3DP across all pediatric disciplines. No single field or single procedure dominated, with over 40 unique conditions described by the articles reviewed (Table 3). Manufacturing variables reflected similar diversity, with over 25 software platforms and 20 different printers used in the articles included in this review. The broad range indicates that universal production standards are not yet in place.
As with all systematic reviews, this study is limited by the papers and data available, subject to publication bias. Some papers did not provide information on variables we collected (e.g., time, cost, etc.) which may paint an incomplete picture of associated manufacturing variables. The caliber of the compiled studies also limits this review: most studies were retrospective case series, and only six were comparative studies that all showed 3DP devices are better or equivalent to conventional methods (Table 3). Although overall our pediatric systematic review suggests that 3DP models improve care, more case-matched comparative studies are needed. Herein lies the difficulty of analyzing 3DP: the most complex anatomy or rare congenital cases that can especially benefit from 3DP are exactly the unique type of cases that are less likely to have a comparison group.
The breadth of 3DP application uncovered in this systematic review suggests clinicians are just scratching the surface, with significant potential for future patient-specific pediatric applications. One area for expansion is the integration of new imaging modalities. Seventy percent of studies employed computed tomography (CT) to create 3DP devices. Generating 3DP items from other imaging modalities including MRI, ultrasound, and 3D-photography could limit the morbidity of ionizing radiation in children, expanding the utility of 3DP in pediatrics. In addition, close examination of our taxonomy points to areas of further potential growth. Class 4. Materials, especially Shaping devices, offer a unique potential to mold a child’s changing anatomy to potentially avoid morbid surgeries. Unlike their adult counterparts, children have malleable anatomy that allows for molding forces of external materials to influence growth patterns. One prime example is the use of 3DP devices to premold orofacial clefts before surgery to potentially improving outcomes and sparing children from secondary revisions. This hints at the importance of this new category—by harnessing materials to influence growing pediatric anatomy, clinicians could potentially reduce the severity of surgery or avoid surgery all together. This newly identified application is relatively untapped with only six studies found in this systematic review, warranting expanded exploration of this application’s potential.
This study investigates the patient-specific uses of 3DP in pediatric populations and identifies a new taxonomy of use. This taxonomy illustrates the diversity of 3DP applications and challenges clinicians to integrate it into their own practice to provide individualized approaches to their patients’ problems. Furthermore, this study shows there is little standardization of these objects across disciplines. With this rapid expansion of 3DP, and the exciting potential for advanced manufacturing techniques, there is a need for a structured, regulated production process to ensure the safety and credibility of 3DP objects. Standardization may also allow for more streamlined and easily accessible production, further empowering clinicians to become actively involved in the process of 3DP patient-specific models and innovating further applications in treating pediatric patients.
Conclusion
Three-dimensional printing (3DP) is transforming medicine with customized patient-specific models that are especially applicable for pediatric patients with smaller anatomy, unusual congenital and acquired defects, and less room for error. Our systematic review uniquely focused on patient-specific 3DP applications in this special population, and we identified a new taxonomy with four distinct, comprehensive classes of Three-Dimensional Printing in Medicine: Teaching, Developing, Procedures, Materials. These applications of 3DP are particularly advantageous in pediatric medicine, as patient-specific 3DP models educate patients and families; expose underlying anatomy in complex congenital cases to help develop clinical plans; improve procedural accuracy, safety, and efficiency; and create rapid, cost-effective materials that accommodate a growing child. This taxonomy helps categorize what is currently available, helping to promote further innovation and support incorporation of this individualized, patient-centered care into the field of pediatrics.
References
Jacobs, C. A. & Lin, A. Y. A new classification of three-dimensional printing technologies: systematic review of three-dimensional printing for patient-specific craniomaxillofacial surgery. Plast. Reconstr. Surg. 139, 1211–1220 (2017).
Malik, H. H. et al. Three-dimensional printing in surgery: a review of current surgical applications. J. Surg. Res. 199, 512–522 (2015).
Pucci, J. U., Christophe, B. R., Sisti, J. A. & Connolly, E. S. Jr Three-dimensional printing: technologies, applications, and limitations in neurosurgery. Biotechnol. Adv. 35, 521–529 (2017).
Bauermeister, A. J., Zuriarrain, A. & Newman, M. I. Three-dimensional printing in plastic and reconstructive surgery: a systematic review. Ann. Plast. Surg. 77, 569–576 (2016).
Yao, R. et al. Three-dimensional printing: review of application in medicine and hepatic surgery. Cancer Biol. Med. 13, 443–451 (2016).
Crafts, T. D. et al. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol. Head Neck Surg. 156, 999–1010 (2017).
Eltorai, A. E., Nguyen, E. & Daniels, A. H. Three-dimensional printing in orthopedic surgery. Orthopedics 38, 684–687 (2015).
Sugrue, C. M., Joyce, C. W. & Carroll, S. M. Levels of Evidence in Plastic and Reconstructive Surgery Research: Have We Improved Over the Past 10 Years? Plast. Reconstr. Surg. Glob. Open. 7 (2019).
Weissler, J. M., Sosin, M., Dorafshar, A. H. & Garcia, J. R. Combining virtual surgical planning, intraoperative navigation, and 3-dimensional printing in prosthetic-based bilateral microtia reconstruction. J. Oral Maxillofac. Surg. 75, 1491–1497 (2017).
Watson, J. & Hatamleh, M. M. Complete integration of technology for improved reproduction of auricular prostheses. J. Prosthet. Dent. 111, 430–436 (2014).
Hatamleh, M. M. & Watson, J. Construction of an implant-retained auricular prosthesis with the aid of contemporary digital technologies: a clinical report. J. Prosthodont. 22, 132–136 (2013).
Zheng, Y., Zhang, D., Qin, T. & Wu, G. Correction of nasal deformity in infants with unilateral cleft lip and palate using multiple digital techniques. J. Prosthet. Dent. 115, 788–791 (2016).
Jeon, B. et al. Fabrication of three-dimensional scan-to-print ear model for microtia reconstruction. J. Surg. Res. 206, 490–497 (2016).
Nuseir, A. et al. Improved construction of auricular prosthesis by digital technologies. J. Craniofac. Surg. 26, e502–e505 (2015).
Chen, H. Y. et al. Pursuing mirror image reconstruction in unilateral microtia: customizing auricular framework by application of three-dimensional imaging and three-dimensional printing. Plast. Reconstr. Surg. 139, 1433–1443 (2017).
Zuniga, J. et al. Cyborg beast: a low-cost 3d-printed prosthetic hand for children with upper-limb differences. BMC Res. Notes 8, 10 (2015).
Tam, M. D., Laycock, S. D., Bell, D. & Chojnowski, A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J. Radio. Case Rep. 6, 31–37 (2012).
Trout, A. T. et al. 3D printed pathological sectioning boxes to facilitate radiological-pathological correlation in hepatectomy cases. J. Clin. Pathol. 70, 984–987 (2017).
Xu, G. et al. Three-dimensional-printed upper limb prosthesis for a child with traumatic amputation of right wrist: a case report. Medicine 96, e9426 (2017).
Krauel, L. et al. Use of 3D prototypes for complex surgical oncologic cases. World J. Surg. 40, 889–894 (2016).
Zuniga, J. M. P., Peck, J. O. T. L. C. H. T., Srivastava, R. M. S. C. P. O., Katsavelis, D. P. & Carson, A. B. S. An open source 3D-printed transitional hand prosthesis for children. JPO J. Prosthet. Orthot. 28, 103–108 (2016).
Zuniga, J. M., et al. Functional changes through the usage of 3D-printed transitional prostheses in children. Disabil. Rehabil. Assist. Technol. 14, 68–74 (2017).
Morrison, R. J. et al. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 7, 285ra264 (2015).
Vodiskar, J., Kutting, M., Steinseifer, U., Vazquez-Jimenez, J. F. & Sonntag, S. J. Using 3D physical modeling to plan surgical corrections of complex congenital heart defects. Thorac. Cardiovasc. Surg. 65, 31–35 (2017).
Valverde, I. et al. 3D printed models for planning endovascular stenting in transverse aortic arch hypoplasia. Catheter Cardiovasc. Inter. 85, 1006–1012 (2015).
Olivieri, L. J. et al. “Just-in-time” simulation training using 3-D printed cardiac models after congenital cardiac surgery. World J. Pediatr. Congenit. Heart Surg. 7, 164−168 (2016).
Biglino, G., Moharem-Elgamal, S., Lee, M., Tulloh, R. & Caputo, M. The perception of a three-dimensional-printed heart model from the perspective of different stakeholders: a complex case of truncus arteriosus. Front. Pediatr. 5, 209 (2017).
Sahayaraj, R. A., Ramanan, S., Subramanyan, R. & Cherian, K. M. 3D printing to model surgical repair of complex congenitally corrected transposition of the great arteries. World J. Pediatr. Congenit. Heart Surg. 10, 373–375 (2017).
Tricot, M., Duy, K. T. & Docquier, P. L. 3D-corrective osteotomy using surgical guides for posttraumatic distal humeral deformity. Acta Orthop. Belg. 78, 538–542 (2012).
Byrne, A. M., Impelmans, B., Bertrand, V., Van Haver, A. & Verstreken, F. Corrective osteotomy for malunited diaphyseal forearm fractures using preoperative 3-dimensional planning and patient-specific surgical guides and implants. J. Hand Surg. Am. 42, 836.e1–836.e12 (2017).
Soleman, J., Thieringer, F., Beinemann, J., Kunz, C. & Guzman, R. Computer-assisted virtual planning and surgical template fabrication for frontoorbital advancement. Neurosurg. Focus 38, E5 (2015).
Rogers-Vizena, C. R., Sporn, S. F., Daniels, K. M., Padwa, B. L. & Weinstock, P. Cost-benefit analysis of three-dimensional craniofacial models for midfacial distraction: a pilot study. Cleft Palate Craniofac. J. 54, 612–617 (2017).
Faber, J., Berto, P. M. & Quaresma, M. Rapid prototyping as a tool for diagnosis and treatment planning for maxillary canine impaction. Am. J. Orthod. Dentofac. Orthop. 129, 583–589 (2006).
Song, Z. L., Feng, C. K., Chiu, F. Y. & Liu, C. L. The clinical significance of rapid prototyping technique in complex spinal deformity surgery—case sharing and literature review. Formos. J. Musculoskelet. Disord. 4, 88–93 (2013).
Pacione, D., Tanweer, O., Berman, P. & Harter, D. H. The utility of a multimaterial 3D printed model for surgical planning of complex deformity of the skull base and craniovertebral junction. J. Neurosurg. 125, 1194–1197 (2016).
Valverde, I. et al. Three-dimensional patient-specific cardiac model for surgical planning in Nikaidoh procedure. Cardiol. Young-. 25, 698–704 (2015).
Resnick, C. M. Precise osteotomies for mandibular distraction in infants with Robin sequence using virtual surgical planning. Int. J. Oral Maxillofac. Surg. 47, 35–43 (2018).
Shen, C., Yao, C. A., Magee, W. 3rd, Chai, G. & Zhang, Y. Presurgical nasoalveolar molding for cleft lip and palate: the application of digitally designed molds. Plast. Reconstr. Surg. 135, 1007e–1015e (2015).
Fiaschi, P. et al. Surgical results of cranioplasty with a polymethylmethacrylate customized cranial implant in pediatric patients: a single-center experience. J. Neurosurg. Pediatr. 17, 705–710 (2016).
Choi, J. W., Koh, K. S., Hong, J. P., Hong, S. H. & Ra, Y. One-piece frontoorbital advancement with distraction but without a supraorbital bar for coronal craniosynostosis. J. Plast. Reconstr. Aesthet. Surg. 62, 1166–1173 (2009).
Kim, Y. O. et al. Cranial growth after distraction osteogenesis of the craniosynostosis. J. Craniofac. Surg. 19, 45–55 (2008).
Gong, X. & Yu, Q. Correction of maxillary deformity in infants with bilateral cleft lip and palate using computer-assisted design. Oral Surg. Oral Med. Oral Pathol. Oral Radio. 114, S74–S78 (2012).
Sun, H. et al. Error analysis of a CAD/CAM method for unidirectional mandibular distraction osteogenesis in the treatment of hemifacial microsomia. Br. J. Oral Maxillofac. Surg. 51, 892–897 (2013).
Shi, L. et al. Surgical guide assistant mandibular distraction osteogenesis and sagittal split osteotomy in the treatment of hemifacial microsomia. J. Craniofac. Surg. 26, 498–500 (2015).
Wang, C. et al. Surgical template to minimize the damage of tooth buds in young children with mandibular distraction osteogenesis. J. Craniofac. Surg. 27, 1732–1734 (2016).
Chen, Y. et al. Three-dimensional preoperative design of distraction osteogenesis for hemifacial microsomia. J. Craniofac. Surg. 25, 184–188 (2014).
Yu, Q. et al. A novel technique for presurgical nasoalveolar molding using computer-aided reverse engineering and rapid prototyping. J. Craniofac. Surg. 22, 142–146 (2011).
Verweij, J. P. et al. Autotransplantation of premolars with a 3-dimensional printed titanium replica of the donor tooth functioning as a surgical guide: proof of concept. J. Oral Maxillofac. Surg. 74, 1114–1119 (2016).
Graf, S., Cornelis, M. A., Hauber Gameiro, G. & Cattaneo, P. M. Computer-aided design and manufacture of hyrax devices: can we really go digital? Am. J. Orthod. Dentofac. Orthop. 152, 870–874 (2017).
Wei, Y., Li-Tsang, C. W. P., Liu, J., Xie, L. & Yue, S. 3D-printed transparent facemasks in the treatment of facial hypertrophic scars of young children with burns. Burns 43, e19–e26 (2017).
Paeng, J. Y., Lee, J. H., Lee, J. H. & Kim, M. J. Condyle as the point of rotation for 3-D planning of distraction osteogenesis for hemifacial microsomia. J. Craniomaxillofac. Surg. 35, 91–102 (2007).
Emodi, O., Shilo, D., Israel, Y. & Rachmiel, A. Three-dimensional planning and printing of guides and templates for reconstruction of the mandibular ramus and condyle using autogenous costochondral grafts. Br. J. Oral Maxillofac. Surg. 55, 102–104 (2017).
Mourits, D. L. et al. 3D orbital reconstruction in a patient with microphthalmos and a large orbital cyst—a case report. Ophthalmic Genet. 37, 233–237 (2016).
Serlo, W., Ashammakhi, N., Tormala, P. & Waris, T. A new technique for correction of trigonocephaly in an infant: application of an absorbable endocranial plate. Childs Nerv. Syst. 16, 595–597 (2000).
Deshmukh, T. R., Kuthe, A. M., Chaware, S. M., Bagaria, V. & Ingole, D. S. A novel rapid prototyping and finite element method-based development of the patient-specific temporomandibular joint implant. Comput. Methods Biomech. Biomed. Eng. 15, 363–370 (2012).
Kaur, H. et al. An alternate vista in rehabilitation of cranial defects: combining digital and manual techniques to fabricate a hybrid cranioplast. J. Craniofac. Surg. 26, 1313–1315 (2015).
VanKoevering, K. K. et al. Antenatal three-dimensional printing of aberrant facial anatomy. Pediatrics 136, e1382–e1385 (2015).
Carlos, C., Parkes, W. & James, A. L. Application of 3-dimensional modeling to plan totally endoscopic per-meatal drainage of petrous apex cholesterol granuloma. Otolaryngol. Head Neck Surg. 153, 1074–1075 (2015).
Mao, Z., Cui, Y. & Wang, H. Application of computer-aided design combined with 3D printing technology in the treatment of infantile congenital maxillomandibular fusion. Int. J. Clin. Exp. Med. 9, 18941–18945 (2016).
Pang, N. S., Choi, Y. K., Kim, K. D. & Park, W. Autotransplantation of an ectopic impacted premolar with sinus lift and allogenic bone graft. Int. Endod. J. 44, 967–975 (2011).
Lee, Y. et al. Autotransplantation of mesiodens for missing maxillary lateral incisor with cone-beam CT-fabricated model and orthodontics. Int. Endod. J. 47, 896–904 (2014).
Kim, M. S., Lee, H. S., Nam, O. H. & Choi, S. C. Autotransplantation: a reliable treatment modality for severely malpositioned teeth. J. Clin. Pediatr. Dent. 41, 388–391 (2017).
Zopf, D. A., Hollister, S. J., Nelson, M. E., Ohye, R. G. & Green, G. E. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 368, 2043–2045 (2013).
Shahbazian, M., Wyatt, J., Willems, G. & Jacobs, R. Clinical application of a stereolithographic tooth replica and surgical guide in tooth autotransplantation. Virtual Phys. Prototyp. 7, 211–218 (2012).
Salles, F. et al. Complete and isolated congenital aglossia: case report and treatment of sequelae using rapid prototyping models. Oral Surg. Oral Med. Oral Pathol. Oral Radio. Endod. 105, e41–e47 (2008).
Wang, W., Duan, J., Wang, Q. & Kuang, W. Complex reconstruction of facial deformity and function after severe gunshot injury: one case report. Int. J. Clin. Exp. Med. 8, 1427–1433 (2015).
Wu, C. T., Lee, S. T., Chen, J. F., Lin, K. L. & Yen, S. H. Computer-aided design for three-dimensional titanium mesh used for repairing skull base bone defect in pediatric neurofibromatosis type 1. A novel approach combining biomodeling and neuronavigation. Pediatr. Neurosurg. 44, 133–139 (2008).
Byun, C. et al. Endodontic treatment of an anomalous anterior tooth with the aid of a 3-dimensional printed physical tooth model. J. Endod. 41, 961–965 (2015).
Nahumi, N., Shohet, M. R., Bederson, J. B. & Elahi, E. Frontorbital fibrous dysplasia resection and reconstruction with custom polyetherlatone alloplast. J. Craniofac. Surg. 26, e720–e722 (2015).
Strbac, G. D. et al. Guided autotransplantation of teeth: a novel method using virtually planned 3-dimensional templates. J. Endod. 42, 1844–1850 (2016).
Cho, M. J., Kane, A. A., Hallac, R. R., Gangopadhyay, N. & Seaward, J. R. Liquid latex molding: a novel application of 3D printing to facilitate flap design. Cleft Palate Craniofac. J. 54, 453–456 (2017).
Janakiraman, N., Vaziri, H., Safavi, K., Nanda, R. & Uribe, F. Management of severely impacted mandibular canines and congenitally missing mandibular premolars with protraction of autotransplanted maxillary premolar. Am. J. Orthod. Dentofac. Orthop. 150, 339–351 (2016).
Ren, X. C., Li, Y. F., Liu, Y. & Zhu, S. S. Mandibular symphyseal midline distraction osteogenesis for micrognathia associated with aglossia and situs inversus totalis. Int. J. Oral Maxillofac. Surg. 46, 1346–1351 (2017).
Hodges, M. M. et al. Massive facial teratoma managed with the ex utero intrapartum treatment (EXIT) procedure and use of a 3-dimensional printed model for planning of staged debulking. J. Pediatr. Surg. Case Rep. 17, 15–19 (2017).
Lee, J. W., Choi, B. J., Nam, O. H. & Kwon, Y. D. Minimal invasive treatment using patient-specific template for mandibular fractures in children: “Wing-splint” by CAD/CAM technology. Br. J. Oral Maxillofac. Surg. 54, 1140–1141 (2016).
Fernandes, N., van den Heever, J., Sykes, L. & Kluge, H. Nasal reconstruction of a patient with complete congenital arhinia: a clinical report. J. Prosthet. Dent. 116, 924–927 (2016).
Park, J. M., Tatad, J. C., Landayan, M. E., Heo, S. J. & Kim, S. J. Optimizing third molar autotransplantation: applications of reverse-engineered surgical templates and rapid prototyping of three-dimensional teeth. J. Oral Maxillofac. Surg. 72, 1653–1659 (2014).
Rose, A. S. et al. Pre-operative simulation of pediatric mastoid surgery with 3D-printed temporal bone models. Int. J. Pediatr. Otorhinolaryngol. 79, 740–744 (2015).
Wolfswinkel, E. M., Imahiyerobo, T. A., McComb, J. G., Sanchez-Lara, P. A. & Urata, M. M. Proteus syndrome with a cranial intraosseous lipoma. J. Craniofac. Surg. 28, e771–e773 (2017).
Darwood, A. et al. Re-thinking 3D printing: a novel approach to guided facial contouring. J. Craniomaxillofac. Surg. 43, 1256–1260 (2015).
Verweij, J. P., Anssari Moin, D., Wismeijer, D. & van Merkesteyn, J. P. R. Replacing heavily damaged teeth by third molar autotransplantation with the use of cone-beam computed tomography and rapid prototyping. J. Oral Maxillofac. Surg. 75, 1809–1816 (2017).
Man, Q. W., Jia, J., Liu, K., Chen, G. & Liu, B. Secondary reconstruction for mandibular osteoradionecrosis defect with fibula osteomyocutaneous flap flowthrough from radial forearm flap using stereolithographic 3-dimensional printing modeling technology. J. Craniofac. Surg. 26, e190–e193 (2015).
Wan, W. B., Shi, P. F. & Li, S. G. Segmentation, surface rendering, and surface simplification of 3-D skull images for the repair of a large skull defect. J. Electron. Imaging 18, 043003 (2009).
Kim, J. C. & Hong, I. P. Split-rib cranioplasty using a patient-specific three-dimensional printing model. Arch. Plast. Surg. 43, 379–381 (2016).
Kfir, A., Telishevsky-Strauss, Y., Leitner, A. & Metzger, Z. The diagnosis and conservative treatment of a complex type 3 dens invaginatus using cone beam computed tomography (CBCT) and 3D plastic models. Int. Endod. J. 46, 275–288 (2013).
Engel, M., Hoffmann, J., Castrillon-Oberndorfer, G. & Freudlsperger, C. The value of three-dimensional printing modelling for surgical correction of orbital hypertelorism. Oral Maxillofac. Surg. 19, 91–95 (2015).
Olszewski, R. & Reychler, H. Three-dimensional surgical guide for frontal-nasal-ethmoid-vomer disjunction in Le Fort III osteotomy. J. Craniofac. Surg. 22, 1791–1792 (2011).
Kuijten, M. M. P., Remmers, J. S., Mourits, D. L., de Graaf, P. & Hartong, D. T. Three-dimensionally printed conformers for treatment of congenital anophthalmos. Ophthalmic Plast. Reconstr. Surg. 33, 394–395 (2017).
Danelson, K. A., Gordon, E. S., David, L. R. & Stitzel, J. D. Using a three dimensional model of the pediatric skull for pre-operative planning in the treatment of craniosynostosis—biomed 2009. Biomed. Sci. Instrum. 45, 358–363 (2009).
Wiedermann, J. P., Joshi, A. S., Jamshidi, A., Conchenour, C. & Preciado, D. Utilization of a submental island flap and 3D printed model for skull base reconstruction: infantile giant cranio-cervicofacial teratoma. Int. J. Pediatr. Otorhinolaryngol. 92, 143–145 (2017).
Robiony, M. et al. Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J. Oral Maxillofac. Surg. 65, 1198–1208 (2007).
LoPresti, M., Daniels, B., Buchanan, E.P., Monson, L. & Lam, S. Virtual surgical planning and 3D printing in repeat calvarial vault reconstruction for craniosynostosis: technical note. J. Neurosurg. Pediatr. 19, 490−494 (2017).
Yang, M. et al. Application of 3D rapid prototyping technology in posterior corrective surgery for Lenke 1 adolescent idiopathic scoliosis patients. Medicine 94, e582 (2015).
Pan, Y., Lu, G. H., Kuang, L. & Wang, B. Accuracy of thoracic pedicle screw placement in adolescent patients with severe spinal deformities: a retrospective study comparing drill guide template with free-hand technique. Eur. Spine J. 27, 319–326 (2018).
Lu, S. et al. Accuracy and efficacy of thoracic pedicle screws in scoliosis with patient-specific drill template. Med. Biol. Eng. Comput. 50, 751–758 (2012).
Karlin, L., Weinstock, P., Hedequist, D. & Prabhu, S. P. The surgical treatment of spinal deformity in children with myelomeningocele: the role of personalized three-dimensional printed models. J. Pediatr. Orthop. B 26, 375–382 (2017).
Paiva, W. S., Amorim, R., Bezerra, D. A. & Masini, M. Application of the stereolithography technique in complex spine surgery. Arq. Neuropsiquiatr. 65, 443–445 (2007).
Sakai, T. et al. Pediatric patient with incidental Os odontoideum safely treated with posterior fixation using rod-hook system and preoperative planning using 3D printer: a case report. J. Neurol. Surg. A Cent. Eur. Neurosurg. 78, 306–309 (2017).
Werner, H., Lopes, J., Tonni, G. & Araujo Junior, E. Physical model from 3D ultrasound and magnetic resonance imaging scan data reconstruction of lumbosacral myelomeningocele in a fetus with Chiari II malformation. Childs Nerv. Syst. 31, 511–513 (2015).
Xu, N. et al. Reconstruction of the upper cervical spine using a personalized 3D-printed vertebral body in an adolescent with Ewing sarcoma. Spine 41, E50–E54 (2016).
Biglino, G. et al. Piloting the use of patient-specific cardiac models as a novel tool to facilitate communication during cinical consultations. Pediatr. Cardiol. 38, 813–818 (2017).
Biglino, G. et al. Involving patients, families and medical staff in the evaluation of 3D printing models of congenital heart disease. Commun. Med. 12, 157–169 (2015).
Olejnik, P. et al. Utilisation of three-dimensional printed heart models for operative planning of complex congenital heart defects. Kardiol. Pol. 75, 495–501 (2017).
Ngan, E. M. et al. The rapid prototyping of anatomic models in pulmonary atresia. J. Thorac. Cardiovasc. Surg. 132, 264–269 (2006).
Bhatla, P. et al. Utility and scope of rapid prototyping in patients with complex muscular ventricular septal defects or double-outlet right ventricle: does it alter management decisions? Pediatr. Cardiol. 38, 103–114 (2017).
Garekar, S. et al. Clinical application and multidisciplinary assessment of three dimensional printing in double outlet right ventricle with remote ventricular septal defect. World J. Pediatr. Congenit. Heart Surg. 7, 344–350 (2016).
Kappanayil, M., Koneti, N. R., Kannan, R. R., Kottayil, B. P. & Kumar, K. Three-dimensional-printed cardiac prototypes aid surgical decision-making and preoperative planning in selected cases of complex congenital heart diseases: early experience and proof of concept in a resource-limited environment. Ann. Pediatr. Cardiol. 10, 117–125 (2017).
McGovern, E. et al. Clinical application of three-dimensional printing to the management of complex univentricular hearts with abnormal systemic or pulmonary venous drainage. Cardiol. Young-. 27, 1248–1256 (2017).
Sodian, R. et al. Pediatric cardiac transplantation: three-dimensional printing of anatomic models for surgical planning of heart transplantation in patients with univentricular heart. J. Thorac. Cardiovasc. Surg. 136, 1098–1099 (2008).
Sodian, R. et al. Stereolithographic models for surgical planning in congenital heart surgery. Ann. Thorac. Surg. 83, 1854–1857 (2007).
Bhatla, P., Mosca, R. S. & Tretter, J. T. Altering management decisions with gained anatomical insight from a 3D printed model of a complex ventricular septal defect. Cardiol. Young-. 27, 377–380 (2017).
Bharati, A., Garekar, S., Agarwal, V., Merchant, S. A. & Solanki, N. MRA-based 3D-printed heart model-an effective tool in the pre-surgical planning of DORV. BJR Case Rep. 2, 20150436 (2016).
Wilson, C. A. et al. Printed three-dimensional airway model assists planning of single-lung ventilation in a small child. Br. J. Anaesth. 115, 616–620 (2015).
Bhatla, P., Tretter, J. T., Chikkabyrappa, S., Chakravarti, S. & Mosca, R. S. Surgical planning for a complex double-outlet right ventricle using 3D printing. Echocardiography 34, 802–804 (2017).
Simal, I. et al. Three-dimensional custom-made titanium ribs for reconstruction of a large chest wall defect. Eur. J. Pediatr. Surg. Rep. 4, 26–30 (2016).
Kiraly, L., Tofeig, M., Jha, N. K. & Talo, H. Three-dimensional printed prototypes refine the anatomy of post-modified Norwood-1 complex aortic arch obstruction and allow presurgical simulation of the repair. Interact. Cardiovasc. Thorac. Surg. 22, 238–240 (2016).
Jaworski, R., Haponiuk, I., Chojnicki, M., Olszewski, H. & Lulewicz, P. Three-dimensional printing technology supports surgery planning in patients with complex congenital heart defects. Kardiol. Pol. 75, 185 (2017).
Sodian, R. et al. Tissue engineering of vascular conduits: fabrication of custom-made scaffolds using rapid prototyping techniques. Thorac. Cardiovasc. Surg. 53, 144–149 (2005).
Farooqi, K. M. et al. Use of 3-dimensional printing to demonstrate complex intracardiac relationships in double-outlet right ventricle for surgical planning. Circ. Cardiovasc. Imaging 8, e003043 (2015).
Farooqi, K. M., Gonzalez-Lengua, C., Shenoy, R., Sanz, J. & Nguyen, K. Use of a three dimensional printed cardiac model to assess suitability for biventricular repair. World J. Pediatr. Congenit. Heart Surg. 7, 414–416 (2016).
Biglino, G. et al. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: feasibility and acceptability. BMJ Open 5, e007165 (2015).
Storelli, D. A., Bauer, A. S., Lattanza, L. L. & McCarroll, H. R. Jr The use of computer-aided design and 3-dimensional models in the treatment of forearm malunions in children. Tech. Hand Extrem. Surg. 19, 23–26 (2015).
Galvez, J. A. et al. Assessment and planning for a pediatric bilateral hand transplant using 3-dimensional modeling: case report. J. Hand Surg. Am. 41, 341–343 (2016).
Gretsch, K. F. et al. Development of novel 3D-printed robotic prosthetic for transradial amputees. Prosthet. Orthot. Int. 40, 400–403 (2016).
Jeuken, R. M., Hendrickx, R. P. M., Schotanus, M. G. M. & Jansen, E. J. Near-anatomical correction using a CT-guided technique of a forearm malunion in a 15-year-old girl: a case report including surgical technique. Orthop. Traumatol. Surg. Res. 103, 783–790 (2017).
Momeni, A., Chang, B. & Levin, L. S. Technology and vascularized composite allotransplantation (VCA)-lessons learned from the first bilateral pediatric hand transplant. J. Mater. Sci. Mater. Med. 27, 161 (2016).
Zuniga, J. M. et al. The development of a low-cost three-dimensional printed shoulder, arm, and hand prostheses for children. Prosthet. Orthot. Int. 41, 205–209 (2017).
Zheng, P., Xu, P., Yao, Q., Tang, K. & Lou, Y. 3D-printed navigation template in proximal femoral osteotomy for older children with developmental dysplasia of the hip. Sci. Rep. 7, 44993 (2017).
Zheng, P., Yao, Q., Xu, P. & Wang, L. Application of computer-aided design and 3D-printed navigation template in Locking Compression Pediatric Hip Plate(TauMu) placement for pediatric hip disease. Int. J. Comput. Assist. Radio. Surg. 12, 865–871 (2017).
Liu, X. et al. Newly designed foot orthosis for children with residual clubfoot after ponseti casting. J. Prosthet. Orthot. 26, 38−42 (2014).
Cherkasskiy, L. et al. Patient-specific 3D models aid planning for triplane proximal femoral osteotomy in slipped capital femoral epiphysis. J. Child Orthop. 11, 147–153 (2017).
Bellanova, L., Paul, L. & Docquier, P. L. Surgical guides (patient-specific instruments) for pediatric tibial bone sarcoma resection and allograft reconstruction. Sarcoma 2013, 787653 (2013).
Li, Z. et al. Composite artificial semi-knee joint system. Eur. Rev. Med. Pharm. Sci. 18, 1229–1240 (2014).
Holt, A. M., Starosolski, Z., Kan, J. H. & Rosenfeld, S. B. Rapid prototyping 3D model in treatment of pediatric hip dysplasia: a case report. Iowa Orthop. J. 37, 157–162 (2017).
Burzynska, K., Morasiewicz, P. & Filipiak, J. The use of 3D printing technology in the Ilizarov method treatment: pilot study. Adv. Clin. Exp. Med. 25, 1157–1163 (2016).
Ren, X., Yang, L. & Duan, X. J. Three-dimensional printing in the surgical treatment of osteoid osteoma of the calcaneus: a case report. J. Int. Med. Res. 45, 372–380 (2017).
Souzaki, R. et al. Three-dimensional liver model based on preoperative CT images as a tool to assist in surgical planning for hepatoblastoma in a child. Pediatr. Surg. Int. 31, 593–596 (2015).
Soejima, Y. et al. Three-dimensional printing and biotexture modeling for preoperative simulation in living donor liver transplantation for small infants. Liver Transpl. 22, 1610–1614 (2016).
Chick, J. F. B. et al. Three-dimensional printing facilitates successful endovascular closure of a type II Abernethy malformation using an Amplatzer Atrial Septal Occluder Device. Ann. Vasc. Surg. 43, 311.e315–311.e323 (2017).
Ahn, J. J. et al. Use of 3D reconstruction cloacagrams and 3D printing in cloacal malformations. J. Pediatr. Urol. 13, 395.e1–395.e6 (2017).
Souzaki, R. et al. Preoperative surgical simulation of laparoscopic adrenalectomy for neuroblastoma using a three-dimensional printed model based on preoperative CT images. J. Pediatr. Surg. 50, 2112–2115 (2015).
Giron-Vallejo, O. et al. Three-dimensional printed model of bilateral Wilms tumor: a useful tool for planning nephron sparing surgery. Pediatr. Blood Cancer 65 (2018) https://doi.org/10.1002/pbc.26894.
Simonin, A., Martinerie, S., Levivier, M. & Daniel, R. T. Three-dimensional printing of a sinus pericranii model: technical note. Childs Nerv. Syst. 33, 499–502 (2017).
Hadeed, K., Dulac, Y. & Acar, P. Three-dimensional printing of a complex CHD to plan surgical repair. Cardiol. Young-. 26, 1432–1434 (2016).
Rude, K., Thygesen, T. H. & Sorensen, J. A. Reconstruction of the maxilla using a fibula graft and virtual planning techniques. BMJ Case Rep. 2014, bcr2014203601 (2014).
Choy, W. J. et al. Reconstruction of thoracic spine using a personalized 3D-printed vertebral body in adolescent with T9 primary bone tumor. World Neurosurg. 105, 1032.e1013–1032.e1017 (2017).
Kim, D. et al. Sacral reconstruction with a 3D-printed implant after hemisacrectomy in a patient with sacral osteosarcoma: 1-year follow-up result. Yonsei Med. J. 58, 453–457 (2017).
Lau, I. & Sun, Z. Three-dimensional printing in congenital heart disease: a systematic review. J. Med. Radiat. Sci. 65, 226–236 (2018).
Acknowledgements
The authors thank Dr. Assako Holyke, MD, Ph.D., MLIS (Master of Library and Information Sciences) for her assistance in systematic database search including developing search terms and applying syntax to appropriate databases.
Author information
Authors and Affiliations
Contributions
C.A.F. and A.M.S. designed the study, coordinated data collection, acquired data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. W.T.K. conceptualized and designed the study, analyzed and interpreted data, and critically reviewed and revised the manuscript for important intellectual content. A.Y.L. conceptualized and designed the study, supervised data collection, analyzed and interpreted data, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Francoisse, C.A., Sescleifer, A.M., King, W.T. et al. Three-dimensional printing in medicine: a systematic review of pediatric applications. Pediatr Res 89, 415–425 (2021). https://doi.org/10.1038/s41390-020-0991-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0991-6