Abstract
Background
Very low birth weight (VLBW) infants may be at risk for late-onset circulatory collapse (LCC) where otherwise stable infants develop hypotension resistant to vasoactive agents. The risk factors for LCC development are poorly defined, and it has been theorized that it may be in part due to withdrawal from exogenous prenatal steroids. The goal of this study was to define the clinical characteristics of LCC and investigate its association with antenatal steroid administration.
Methods
This is a retrospective cohort study of infants born ≤1500 g. LCC was retrospectively diagnosed in infants requiring glucocorticoids for circulatory instability at >1 week of life. Demographic and clinical characteristics were compared between groups using Mann–Whitney test.
Results
Three hundred and ten infants were included; 19 (6.1%) developed LCC. Infants with LCC were born at a median 4.6 weeks’ lower gestation, 509 g lower birth weight than those without LCC. There was no difference in antenatal steroid delivery between the groups.
Conclusions
LCC occurs in a distinct subset of VLBW infants, suggesting the need for monitoring in this high-risk population. Antenatal steroids did not significantly increase the risk of LCC development in this study.
Impact
-
Late-onset circulatory collapse (LCC) is a life-threatening clinical entity occurring in around 6% in VLBW infants and is likely underdiagnosed in the United States.
-
Targeting specific demographic characteristics such as birth weight (<1000 g) and gestational age at birth (<26 weeks) may allow for early identification of high-risk infants, allowing close monitoring and prompt treatment of LCC.
-
No significant association was found between antenatal steroid administration and LCC development, suggesting that the theoretical risks of antenatal steroids on the fetal HPA axis does not outweigh the benefits of antenatal steroids in fetal lung maturity.
-
To date, no studies characterizing LCC have originated outside of Asia. Therefore, providing a description of LCC in a U.S.-based cohort will provide insight into both its prevalence and presentation to inform clinicians about this potentially devastating disorder and foster early diagnosis and treatment.
-
This study validates LCC characteristics and prevalence previously outlined by Asian studies in a single-center U.S.-based cohort while also identifying potential risk factors for LCC development.
-
This manuscript will provide education for U.S. physicians about the risk factors and clinical presentation of LCC to facilitate early diagnosis and treatment, potentially decreasing neonatal mortality.
-
With prompt recognition and treatment of LCC, infants may have decreased exposure to vasoactive medications that have significant systemic effects.
Similar content being viewed by others
Introduction
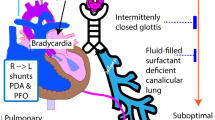
Nearly 1 out of 10 infants delivered in the United States are born preterm (<37 weeks of gestation),1 and worldwide >41,000 preterm infants are born each day.2 Approximately 14% of preterm infants are very low birth weight (VLBW), with birth weights of ≤1500 g.3 A few small studies have described a phenomenon termed late-onset circulatory collapse (LCC) where otherwise medically stable VLBW infants develop hypotension that is resistant to volume expanders and inotropic agents but is responsive to glucocorticoid administration.4,5 It is estimated that around 6% of VLBW infants develop LCC; however, there is little known about the risk factors that may predispose an infant to its development.6 LCC development may be in part due to withdrawal from exogenous steroids compounded by the inability of most preterm neonates to independently synthesize cortisol prior to 30 weeks’ postmenstrual age.7
Despite significant medical advancements in neonatal care, complications from prematurity underlie more than a third of infant deaths, with pulmonary sequelae contributing to >46% of these deaths.8,9 Administration of antenatal corticosteroids to pregnant women at high risk of imminent preterm delivery is currently the standard of care, as it has been shown to reduce neonatal mortality and morbidity by accelerating fetal lung maturity.10,11,12 Despite the clear benefits of antenatal steroid administration, however, some concern has developed around the potential short- and long-term negative effects of exposure to high-dose corticosteroids on the neonatal hypothalamic–pituitary–adrenal (HPA) axis function.13 Early separation of the fetus from the placenta results in an immature HPA axis that subsequently renders the neonate unable to synthesize cortisol.14,15 Although antenatal corticosteroids may initially boost neonatal adrenal function and prevent alterations in blood pressure, when coupled with an already immature HPA axis, the exogenous steroids may further reduce the infant’s inability to synthesize cortisol through the negative feedback loop on the hypothalamus.16
The primary objective of this study was to investigate the association between antenatal steroid administration and LCC development in VLBW infants. As a secondary aim, since all previous studies of LCC originated outside of the United States, we sought to define the characteristics of LCC onset and risk factors in a single-center U.S. cohort of VLBW infants. The goal of the study was to aid in the identification of infants at high risk of developing LCC, thus enabling earlier recognition of its symptoms and allowing for more prompt treatment and potentially improved patient outcomes.
Methods
This retrospective single-center cohort study was approved by the University of Nebraska Medical Center Institutional Review Board. Infants born at ≤1500 g who were admitted to either the University of Nebraska Medical Center neonatal intensive care unit (NICU) or Children’s Hospital and Medical Center NICU between October 1, 2011 and March 1, 2019 were enrolled in this study. Infants were excluded if they died or were transferred out within 1 week of life, were diagnosed with congenital adrenal hypoplasia, panhypopituitarism, congenital cardiac anomalies, or if their hypotension was found to be due to another common cause of circulatory collapse, such as necrotizing enterocolitis, or culture-positive sepsis.
Although the diagnostic criteria for LCC have not yet been fully defined, the Japanese Study Group for Neonatal Endocrinology has suggested the following diagnostic criteria: (1) LCC occurs outside the transitional period; (2) a stable period exists prior to the onset of LCC; (3) there are no apparent causes such as sepsis, massive bleeding, or necrotizing enterocolitis prior to the onset of LCC; (4) hypotension and/or oliguria with sudden onset; and (5) hypotension and/or oliguria resistant to intravenous volume expanders and inotropes.5 Although treatment was at the discretion of the attending provider, the general guidelines of the units during the study period were to treat with vasopressors for mean blood pressures lower than gestational age in weeks if also combined with signs of decreased perfusion. Hydrocortisone was used for low blood pressure not responding to vasoactive agents. During the study period, prophylactic steroids for prevention of bronchopulmonary dysplasia or hypotension were not being used in the units studied. For this study, LCC diagnosis was considered in infants who had previously demonstrated cardiovascular stability off of glucocorticoids but then required hydrocortisone administration to correct an unexplained hypotensive episode after postnatal day 6 (day of birth as day zero). LCC diagnosis was confirmed by the presence of hypotension and oliguria was recorded if present.
Data abstracted from the electronic medical record included demographic data including maternal age and parity, as well as neonatal gestational age at birth, birth weight, sex, and race. For antenatal steroid administration, the type, number of doses, and number of days between the last dose and delivery were recorded. Any postnatal steroids received in the first week of life were categorized as “early,” and the duration and timing of administration were recorded. Vasoactive medication (e.g., dopamine, dobutamine, epinephrine) usage during the first week of life was considered “early” and was recorded as a binary term. Vasopressors used after the first week of life were considered “late” and were also recorded as a binary term.
The LCC time of onset was defined as the start of hypotension requiring hydrocortisone with or without vasopressor administration. The start time and duration of hydrocortisone administration were recorded. Additional data collected at the time of LCC onset included serum electrolyte (sodium, potassium, and glucose) and cortisol levels, as well as systolic, diastolic, and mean arterial blood pressures. The most abnormal electrolyte and blood pressure value within 24 h of LCC onset was recorded. In addition, oliguria was recorded as a binary term, defined as urine output ≤0.5 mL/kg/h averaged over a 24-h period. Lastly, the age at LCC onset as well as survival of the LCC hypotensive event, survival to discharge, length of NICU hospitalization, and concurrent illnesses were recorded.
A subgroup of infants with LCC received hydrocortisone early on in their clinical decompensation and thus did not require vasopressors at the time of LCC onset. This group was termed “LCC recovered with hydrocortisone alone.” To provide some understanding whether these two populations were within the same spectrum of LCC or separate pathophysiology, the clinical characteristics of the LCC without vasopressors group were compared to the subset of infants with LCC who required vasopressors. Demographic data, antenatal steroid administration, presence of oliguria, blood pressure, and electrolyte and cortisol levels were compared to evaluate any potential clinical differences in LCC onset or outcomes between the two groups.
Descriptive statistics include medians, minimums, and maximums for continuous data and counts and percentages for categorical data. Mann–Whitney test was used to compare continuous variables between the dichotomous groups, and Fisher’s exact test was used to compare categorical data. A multivariable logistic regression analysis was performed to determine whether gestational age and birth weight as continuous variables were independent risk factors for LCC development. In addition, regression models were analyzed to determine whether early vasopressor use or early steroid use was an independent risk factor from gestational age and birth weight. All analyses were done in SAS 9.4, and p < 0.05 was considered statistically significant.
Results
Study population
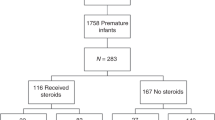
During the study period, 411 infants were born with a birth weight ≤1500 g and were evaluated for inclusion in this study (Fig. 1). Of these, 101 infants met exclusion criteria, leaving 310 infants for analysis. Nineteen infants were retrospectively diagnosed with LCC and were compared to the remaining 291 infants who did not appear to experience LCC, resulting in a prevalence of 6.1% in this population. Eight out of 19 LCC infants recovered without vasopressors, and the remaining 11 received both vasopressors and hydrocortisone.
Demographics and characteristics of infants with LCC
Demographic data compared between LCC and non-LCC infants revealed significant differences in gestational age, birth weight, and length of hospitalization (Table 1). The distribution of gestational age at birth and birth weight for LCC versus non-LCC infants are shown in Figs. 2 and 3, respectively. LCC developed in 13.4% of infants with a birth weight <1000 g and 18.2% of infants born at ≤26 weeks of gestation at birth. Multivariable logistic regression analysis demonstrated that gestational age and birth weight are both independent risk factors for LCC development (p = 0.03 for each). After adjusting for birth weight, the odds of LCC for infants 1 week older is 0.68 times the odds (95% confidence interval 0.48–0.96, p = 0.027) of LCC for infants 1 week younger. Owing to concerns for collinearity between gestational age and birth weight, correlation between the two variables was assessed and was 0.74 (a correlation value >0.8 would suggest a level of multicollinearity for which the two variables could not be used together in the regression analysis). In addition, the two variables were assessed in separate logistic regression models and the odds ratios in relation to LCC were similar to the odds ratios when both were in the model together.
No significant differences were found between race, gender, survival to discharge, and maternal demographics in these populations. With regard to early markers of illness, 4 (21.1%) infants with LCC required steroids within the first week of life compared to 21 (7.2%) of non-LCC infants. In addition, 6 (31.6%) LCC infants required vasopressors within the first week of life compared to 39 (13.4%) non-LCC infants. However, after adjusting for gestational age and birth weight in a multivariable logistic regression model, neither early vasopressor usage nor early postnatal steroids were independently associated with LCC development (p = 0.75 and p = 0.47, respectively).
LCC recovered with hydrocortisone alone
Analysis of the LCC population revealed that there was a subset of patients who experienced LCC and were treated with hydrocortisone but did not receive vasopressors for LCC recovery. There were no significant differences in demographics or LCC characteristics between the infants with LCC who recovered without vasopressors and those who required vasopressors (Table 2).
LCC characteristics
LCC occurred at a median age of 22 (8, 57) days of life. In the 21.1% (n = 4/19) of LCC patients who received steroids within the first week of life, the median age of LCC onset was 30 (23, 57) days of life compared to a median age of 19 (8, 37) days of life in neonates who did not receive steroids within the first week of life. All infants were started on hydrocortisone therapy for LCC reversal, with the median duration of steroids lasting 5 (1, 30) days. One of the 19 infants (5.2%) died as a result of the LCC event. For the entire LCC group, the median sodium level at the start of LCC treatment was 134 mEq/L (123, 146), the median potassium level was 6.1 mEq/L (2.4, 10.3), and the median glucose level was 93 mg/dL (30, 317). In the 6 out of 19 (31.6%) patients who had cortisol levels recorded during the time of LCC onset, the median level was 3.95 mcg/dL (1.9, 15). Oliguria was noted in 42.1% (n = 8/19) of LCC infants at LCC onset. There were no significant differences in electrolytes, oliguria, or blood pressures between infants with LCC who recovered with vasopressors and those who recovered without vasopressors.
Antenatal steroid administration
When comparing antenatal steroid administration between the groups, 100% (n = 18) of LCC infants received antenatal steroids compared to 87.9% (n = 240) of non-LCC infants (p = 0.239). Both the LCC infants and non-LCC infants received a median of 2 (1, 4) antenatal steroid doses prior to delivery, showing no statistical difference between the groups. Of those receiving steroids (n = 258), 99.2% (n = 256) of women received betamethasone and 0.8% (n = 2) of women received dexamethasone for antenatal steroid treatment. There was no significant difference between the LCC infants and non-LCC infants in the median number of days between the last dose of antenatal steroids and delivery. There was a mean of 3 (0, 21) days for LCC infants and 4 (0, 44) days for non-LCC infants.
Discussion
LCC is a distinct form of clinical decompensation that may occur in VLBW infants after the first week of life. In this study, 6% of VLBW infants developed LCC, emphasizing the importance of recognizing this as a life-threatening clinical diagnosis. In addition to increased mortality, these infants may also be at higher risk for long-term neurodevelopmental impairment and cerebral palsy.17,18 Infants who develop LCC have a characteristic demographic profile, with the incidence of LCC doubling to 13% in infants with birth weight <1000 g and more than tripling to 19% in infants born at gestational age of ≤26 weeks.
This is the first description of LCC in a North American cohort of VLBW infants. LCC is currently not a focus of discussion in neonatal medicine in the United States; however, this study demonstrates that its prevalence is equally as high in the U.S. as it is in the previous studies from throughout Asia.4,5,6 Educating U.S. physicians about LCC and enabling them to detect infants at a high risk for developing LCC is crucial to allow for proper treatment of this condition. Previous studies of LCC have demonstrated clinical improvement in this population within 4 h of initiation of glucocorticoid therapy,19 so earlier prospective identification and treatment of LCC may potentially reduce neonatal morbidity or mortality.
The optimal duration of glucocorticoid therapy for LCC has not been established. A recent review suggested treating LCC in a similar manner to early vasopressor-resistant hypotension—steadily weaning as tolerated, with a slow taper only in those who continue on hydrocortisone for >3 days.20 In practice, though, many infants with LCC are treated with long tapering courses of hydrocortisone. For example, the median hydrocortisone treatment duration in a 2015 study was 23 days,19 compared to the relatively short durations seen in the current study population (median 3–10 days). The authors from the 2015 study described a common clinical practice of withdrawing hydrocortisone very slowly to prevent LCC relapse, which likely resulted in the long treatment durations. Contrary to all prior studies that made the diagnosis of LCC prospectively, clinicians in the current study (where LCC is not a commonly diagnosed entity) were likely managing infants in a similar manner to early vasopressor-resistant hypotension and the relatively short durations of hydrocortisone reflect that practice.
Despite differences in therapy, the population and risk factors in the current study are similar to those previously published in the Asian literature. For instance, LCC onset in our population occurred at a median of 21 days of life, which is consistent with previous reports showing median diagnosis of LCC between 16 and 21 days of life.5,19,21 Other characteristics in our population, such as oliguria and electrolyte abnormalities, also appear to be in line with previous reports. Median sodium levels in the current study were 133–134 mEq/L, which is similar to a median of 137 mEq/L described by Shimokaze, et al.19 In addition, in the 65 infants described by Lee et al., only 52% had hyponatremia.5 Although oliguria is a key component of LCC, the majority of infants with LCC reported by Shimokaze et al. had a urine output of >1 ml/kg/h for the 8 h prior to LCC onset.19 Despite this appearance of adequate output, however, they reported that most infants had no urine output for several hours prior to diagnosis and there was an average decrease in output of 76% leading up to LCC diagnosis. This variability in metrics used to diagnose oliguria is likely one reason why the current study had rates of oliguria as low as 37–63%.
This study shows that infants who develop LCC tend to be of the lowest gestational age at birth and birth weight, supporting previous reports of increasing LCC incidence with shorter gestation and lower birth weight,5,18,19,21,22 with some studies demonstrating rates as high as 33% at 24 weeks of gestation and 57% in infants born at <500 g.5 Our study demonstrated significant increases in LCC incidence in infants with a birth weight <1000 g and those born at ≤26 weeks of gestation at birth, separating these high-risk infants early in their hospital stay and therefore allowing for close monitoring and treatment of LCC. The infants in the LCC group had a longer length of stay; however, they also tended to be more ill in the first week after delivery, so the increase in length of stay cannot be solely attributed to the development of LCC but rather the critical demographic nature of this population.
While antenatal steroids have proven vital in aiding in fetal lung maturity,10,11,12 it is unclear how the administration of exogenous steroids to the fetus affects neonatal adrenal gland function.23 Previous studies have shown that infants exposed to exogenous glucocorticoids in utero have reduced markers of HPA axis activity in cord blood and amniotic fluid.24 Other studies have shown that antenatal steroid administration suppresses baseline neonatal cortisol levels during the first postnatal week of life16 and decreases the response to corticotropin-releasing hormone stimulation tests at 2 weeks of age.25 One previous study of LCC incidence demonstrated a weak association between the administration of any antenatal steroids and the diagnosis of LCC,22 though the very low rates of antenatal steroids received by the study population (54–58%) make it unclear whether these results are adaptable to a population with nearly universal administration. Our current study demonstrated no significant differences in the number of prenatal steroid doses administered or number of days between the last dose of antenatal steroids and delivery between LCC and non-LCC infants. While previous studies demonstrate altered HPA function in the first few weeks of life, we showed no significant association with the development of LCC, suggesting that the theoretical risks do not outweigh the benefits of antenatal steroids in fetal lung maturity.10,11,12,24
This study was limited by its retrospective nature and modest sample size. Cortisol levels were obtained at the clinicians’ discretion, so were unavailable in the majority of cases, and the timing and indication for obtaining levels were not standardized in those who had the levels drawn. In addition, since previous studies have stated that LCC occurs at least 1 week after delivery,4,5,19 we used the cutoff of 7 days as a diagnostic criterion in this study. It is important to note, however, that three non-LCC infants required vasopressor and hydrocortisone therapy on day of life 7 and five LCC infants developed LCC in the second week of life, suggesting that there is a gray area in the time of onset. Lastly, the retrospective diagnosis of LCC in this study is different from the previous prospective studies that have been published. As such, prospective studies in the North American population will be necessary to confirm that incidence and characteristics are similar to those published in the previous studies. The retrospective nature of this study adds the risk of missing infants with atypical presentations of LCC as well as potentially misdiagnosing infants with LCC who had other causes for hypotension that were not well documented in the electronic medical record.
LCC is a potentially devastating form of circulatory instability that occurs after 1 week of life in otherwise medically stable VLBW infants. Despite a known effect of antenatal steroids on the neonatal HPA axis, these data suggest little evidence to support the concern that antenatal steroids significantly increase the risk of developing LCC. This study affirms a similar prevalence of LCC in a single-center U.S. NICU compared to the 6% of VLBW infants reported in previous studies.4,5,6 In addition, we found significantly higher rates in infants born at <1000 g or ≤26 weeks of gestational age. These findings distinguish this high-risk population early in their hospital stay, allowing for close monitoring of electrolytes and urine output to identify early signs of LCC prior to sudden circulatory collapse. Future studies evaluating prospective diagnosis and treatment of LCC are warranted and could allow for further clarity in the diagnostic criteria and treatment in an effort to improve patient outcomes and reduce neonatal deaths.
References
Murphy, S. L., Mathews, T. J., Martin, J. A., Minkovitz, C. S. & Strobino, D. M. Annual summary of vital statistics: 2013–2014. Pediatrics 139, e20163239 (2017).
Platt, M. J. Outcomes in preterm infants. Public Health 128, 399–403 (2014).
Numerato, D. et al. Mortality and length of stay of very low birth weight and very preterm infants: a EuroHOPE study. PLoS ONE 10, e0131685 (2015).
Miwa, M., Kusuda, S. & Ikeda, K. Late-onset circulatory collapse in very low-birthweight infants: a Japanese perspective. NeoReviews 10, e381 (2009).
Ji Lee, W. et al. Clinical features of late-onset circulatory collapse in preterm infants. Korean J. Perinatol. 24, 148–157 (2013).
Suzuki, Y. et al. Neonatal factors related to center variation in the incidence of late-onset circulatory collapse in extremely preterm infants. PLoS ONE 13, e0198518 (2018).
Fernandez, E. F. & Watterberg, K. L. Relative adrenal insufficiency in the preterm and term infant. J. Perinatol. 29, 44 (2009).
Matthews, T. J., MacDorman, M. F. & Thoma, M. E. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl Vital Stat. Rep. 64, 1–30 (2015).
Wang, C. H. et al. Analysis of in-hospital neonatal death in the tertiary neonatal intensive care unit in China: a multicenter retrospective study. Chin. Med. J. (Engl.) 129, 2652–2658 (2016).
Schraufnagel, D. E. in Breathing in America: Diseases, Progress, and Hope 197–205 (American Thoracic Society, 2010).
El-Sayed, Y. Y., Borders, A. E. & Gyanfi-Bannerman, C. ACOG Committee Opinion: antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 130, 102–109 (2017).
Bunt, J. et al. The effect in premature infants of prenatal corticosteroids on endogenous surfactant synthesis as measured with stable isotopes. Pediatrics 162, 844–849 (2000).
Wapner, R. J. Antenatal corticosteroids for periviable birth. Semin. Perinatol. 37, 410–413 (2013).
Kawai, M. Late‐onset circulatory collapse of prematurity. Pediatr. Int. 59, 391–396 (2017).
Ng, P. C. et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 89, F119–F126 (2004).
Davis, E. P. et al. Antenatal betamethasone treatment has a persisting influence on infant HPA axis regulation. J. Perinatol. 26, 147–153 (2006).
Yasuoka, K. et al. Late-onset circulatory collapse and risk of cerebral palsy in extremely preterm infants. J. Pediatr. 212, 117–123 (2019).
Nakanishi, H. et al. Clinical characterization and long-term prognosis of neurological development in preterm infants with late-onset circulatory collapse. J. Perinatol. 30, 751–756 (2010).
Shimokaze, T., Akaba, K. & Saito, E. Late-onset glucocorticoid-responsive circulatory collapse in preterm infants: clinical characteristics of 14 patients. Tohoku J. Exp. Med. 235, 241–248 (2015).
Iijima, S. Late-onset glucocorticoid-responsive circulatory collapse in premature infants. Pediatr. Neonatol. 60, 603–610 (2019).
Koyama, N. et al. Clinical features of late-onset circulatory dysfunction in premature infants. Res. Rep. Neonatol. 4, 139 (2014).
Suzuki, Y. et al. Neonatal factors related to center variation in the incidence of late-onset circulatory collapse in extremely preterm infants. PLoS ONE 13, e0198518 (2018).
Waffarn, F. & Davis, E. P. Effects of antenatal corticosteroids on the hypothalamic-pituitary-adrenocortical axis of the fetus and newborn: experimental findings and clinical considerations. Am. J. Obstet. Gynecol. 207, 446–454 (2012).
Tegethoff, M., Pryce, C. & Meinlschmidt, G. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: a systematic review. Endocr. Rev. 30, 753–789 (2009).
Niwa, F. et al. Limited response to CRH stimulation tests at 2 weeks of age in preterm infants born at less than 30 weeks of gestational age. Clin. Endocrinol. 78, 724–729 (2013).
Acknowledgements
We thank the University of Nebraska Medical Center for funding this research.
Consent
Patient consent was not required for this study.
Author information
Authors and Affiliations
Contributions
E.S.P. contributed to study design, data interpretation, and provided critical revisions to the final manuscript. K.C.M. conducted the literature search and data collection and contributed to the study design, data interpretation, and writing of the final manuscript. E.R.L. contributed to the data analysis and interpretation and provided critical revisions to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marinelli, K.C., Lyden, E.R. & Peeples, E.S. Clinical risk factors for the development of late-onset circulatory collapse in premature infants. Pediatr Res 89, 968–973 (2021). https://doi.org/10.1038/s41390-020-0990-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0990-7
This article is cited by
-
Presumed adrenal insufficiency in neonates treated with corticosteroids for the prevention of bronchopulmonary dysplasia
Journal of Perinatology (2022)