Abstract
Background
Most studies of neonatal acute kidney injury (AKI) have focused on the first week following birth. Here, we determined the outcomes and risk factors for late AKI (>7d).
Methods
The international AWAKEN study examined AKI in neonates admitted to an intensive care unit. Late AKI was defined as occurring >7 days after birth according to the KDIGO criteria. Models were constructed to assess the association between late AKI and death or length of stay. Unadjusted and adjusted odds for late AKI were calculated for each perinatal factor.
Results
Late AKI occurred in 202/2152 (9%) of enrolled neonates. After adjustment, infants with late AKI had higher odds of death (aOR:2.1, p = 0.02) and longer length of stay (parameter estimate: 21.9, p < 0.001). Risk factors included intubation, oligo- and polyhydramnios, mild-moderate renal anomalies, admission diagnoses of congenital heart disease, necrotizing enterocolitis, surgical need, exposure to diuretics, vasopressors, and NSAIDs, discharge diagnoses of patent ductus arteriosus, necrotizing enterocolitis, sepsis, and urinary tract infection.
Conclusions
Late AKI is common, independently associated with poor short-term outcomes and associated with unique risk factors. These should guide the development of protocols to screen for AKI and research to improve prevention strategies to mitigate the consequences of late AKI.
Similar content being viewed by others
Introduction
The impact of neonatal acute kidney injury (AKI) is becoming more evident. The AWAKEN study (Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates) has confirmed that AKI is common during NICU admission, occurring in 30% of neonates requiring intravenous fluids for >48 h in the neonatal intensive care unit (NICU). In this cohort, those with at least one episode of AKI at any point in the hospitalization have an increased adjusted length of stay of 9 days and 4.6 times higher independent odds of mortality.1,2
Sick neonates admitted to the NICU have unique risk factors for AKI which may be attributable to immature renal physiology, maternal environment, perinatal events, and iatrogenic insults. The preterm kidney has a short window for postnatal glomerulogenesis and both the preterm and term kidney have an inherently low glomerular filtration rate, predisposing them to AKI. Our group and others have focused on identifying risk factors associated with AKI within the first week after birth.3 However, few studies have assessed the impact of AKI later in their course or after an initial episode of AKI4 despite the long duration of neonatal hospitalizations.
To improve our understanding of the importance of late onset AKI, we evaluated the AWAKEN dataset. The primary goals of the current study were to assess the short-term outcomes (including mortality and length of stay) associated with late AKI (defined as occurring >7 days after birth). In addition, we aimed to identify the antenatal, perinatal, and postnatal risk factors for developing late AKI and the gestational age-specific risk factors for the development of late AKI.
Materials and methods
The AWAKEN study methodology along with a comprehensive description of the 24 participating sites has been published.1 The University of Alabama at Birmingham Institutional Review Board (IRB) approved this collaborative study, and each center received approval from their respective IRBs. The study was registered at ClinicalTrials.gov NCT02443389.
Setting and participants
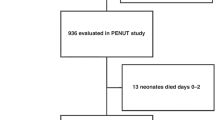
The AWAKEN study was a 3-month retrospective cohort study of patients admitted to a participating NICU between 1 January and 31 March 2014. Data collection began at admission and completed at discharge, death, or 120 days after birth. Figure 1 illustrates the inclusion and exclusion criteria for this study with a final sample of 2152. The outcomes of interest included death, length of stay, and AKI.
Participant flow diagram in late AKI: screening and enrollment. Late AKI was defined as occurring after 7 days after birth using serum creatinine data alone. The No AKI group included those with early AKI but not late AKI and those with insufficient data to diagnose AKI. * Subjects could be excluded for multiple reasons
Neonatal AKI definition
Late AKI was defined according to the modified Kidney Disease: Improving Global Outcomes workgroup AKI definition using serum creatinine alone5,6,7,8 (Supplemental Table S1) and occurring >7 days after birth. The control population included those1 without the diagnosis of AKI (n = 1095),2 with early AKI without late AKI (n = 449) and4 with <2 creatinines after 1 week (n = 608). We compared each patient’s creatinine measurement to the lowest prior serum creatinine and the maximum stage of AKI was used in the analysis. For subjects with early AKI, recovery from the initial episode of AKI was assessed and new creatinine baseline was used in determination of late AKI. All serum creatinine values were ordered by the clinician as per institutional and clinician practice and collected from the medical record.
Data collection
We extracted variables according to their relevance to AKI. All data including admission and discharge diagnoses definitions reflect local practice defined by literature-based definitions.1 Weekly details were collected regarding fluid requirements, electrolytes, blood pressure, and medications. Laboratory monitoring was center-dependent. The data were entered and stored in MediData Rave™, a web-based database housed at Cincinnati Children’s Hospital Medical Center. All admission diagnoses preceded the AKI event, whereas the temporal relationship between the discharge diagnoses to the AKI event was unknown (Supplemental Table S2). Congenital renal anomalies were stratified into mild-to-moderate or severe (Supplemental Table S3). Medications were grouped into the following categories: antimicrobials, methylxanthines, diuretics, vasopressors, and non-steroidal anti-inflammatory drugs (NSAIDs) (Supplemental Table S4). Medication exposure was collected weekly and defined as the number of weeks of each medication class administered prior to the AKI event.
Statistical methods
The categorical variables were analyzed by proportional differences; Χ2 test or Fisher exact test. The non-parametric continuous variables were assessed by Wilcoxon Rank Sum tests (median and interquartile ranges). Continuous variables normally distributed were analyzed using t-tests (means and standard deviations).
The association of late AKI and death within the entire cohort was measured by a logistic regression analysis and was conducted to calculate the odds ratio (ORs) and 95% CI. With the limited number of deaths, the following variables were selected to be included in the model: late AKI, gestational age, mode of delivery, 1-min Apgar, admission for hypoglycemia, inborn errors, congenital heart disease, necrotizing enterocolitits, hypoxic ischemic encephalopathy/seizures, exposure to vasopressors, and NSAIDs. The association between late AKI and length of stay was determined in the whole group and the gestational age strata, linear regression was used to calculate crude parameter estimates and 95% CI. Regression models used a backwards procedure with a significance level of <0.2 of stay. A time-to-event analysis for survival with Kaplan–Meier was used to examine the association of mortality and length of stay with AKI severity in the entire cohort and for each gestational age category with a p < 0.05 considered to be significant.
A generalized estimating equation (GEE) logistic model accounting for clustering by study center was used to estimate the potential risk factors for late AKI (ORs and associated 95% CIs). Models were created for maternal, neonatal, and medication characteristics. Within each analysis, the following were estimated: 1) crude ORs, 2) adjusted to age, ethnicity, and both Apgar scores at 1 and 5 min and then 3) fully adjusted (i.e., for age, ethnicity, and Apgar-1 and 5 in addition to the other variables of interest). In a supplemental analysis, the maternal, neonatal, and medication characteristic variables were considered for inclusion into a single GEE logistic regression model. The most parsimonious model was chosen using a stepwise selection with a model α-level threshold of <0.10 for entry into the model and of 0.10 for remaining in the model. Models were created for the overall study population and for each of the gestational age cohorts. Pearson’s correlation was used to assess the strength of association between median serum creatinine counts and late AKI prevalence by institution.
Results
Demographics
Of the 4270 neonates screened, 2152 were included in the analysis (Fig. 1). The cohort was stratified into 3 gestational age strata: 22–28 weeks (n = 275), 29–35 weeks (n = 954), ≥36 weeks (n = 923). These strata were chosen to maximize sample size, while maintaining clinical relevance and matched the stratification of other AWAKEN analysis. Demographics of the entire cohort and each gestational age category are presented in Table 1.
Late neonatal acute kidney injury
Late AKI occurred in 202/2152 (9%) of the included cohort. Late AKI occurred at a median of 17 days (IQR: 12–32 days) after birth. Within the group who had late AKI, 56/202 (28%) had an early episode of AKI (<7 days) and 24/202 (12%) had >2 episodes of AKI (range: 2–4). The proportion of stage of AKI was consistent within the entire cohort and within the different gestational age cohorts (Supplemental Table S1). The mortality rates between those who had both early and late AKI [12/56 (21%)] were not statistically different than those who had only 1 episode of late AKI [26/178 (15%)] p = 0.22. The average time from early to sentinel late AKI was 37.8 ± 29.4 days. The prevalence of late AKI within each gestational age strata was 29% (n = 80) in 22–28-week group, 5% (n = 51) in the 29–35-week group, and 8% (n = 71) in the ≥36 week group (p < 0.001).
Outcomes: mortality and length of stay
Compared to neonates without late AKI, mortality was higher in the neonates with late AKI in the whole cohort and each gestational age strata. Late AKI was significantly associated with increased mortality in the crude (OR:6.5 (4.0–10.5), p < 0.0001) and adjusted models (aOR:2.1 (1.1–4.0), p = 0.02) for the whole cohort (Table 2). The results did not substantially change when the neonates with insufficient data to diagnose AKI were excluded (Table 2). Gestational age models were not constructed due to the low number of death events within each strata. The more severe stages of late AKI were associated with increased mortality (Fig. 2). Neonates with late AKI had longer length of stay than those without AKI in both the crude (49.0 (44.5–53.4), p < 0.0001) and adjusted (21.9 (18.2–25.7), p = 0.02) models (Table 2). This finding was consistent across all gestational age strata.
Risk factors for late AKI
The risk factors associated with an adjusted odds for late neonatal AKI are summarized in Fig. 3. The adjusted odds to develop late neonatal AKI was analyzed for maternal (Table 3, Supplemental Table S5), neonatal (Table 4) and medications/institutional level (Table 5) exposures. Prevalence of late AKI and median serum creatinine counts was variable across country and type of institution (Supplemental Table S6). In the whole groups intubation, oligo- and polyhydramnios, mild-to-moderate renal anomalies, admission diagnoses of congenital heart disease, necrotizing enterocolitis, and surgical need, were all neonatal factors associated with an increased odds of developing late AKI. Discharge diagnoses of patent ductus arteriosus, necrotizing enterocolitis, sepsis, and urinary tract infection were also associated with an increased odds of late AKI. Medication exposures such as diuretics and vasopressors, and NSAIDs were associated with an increased odds of late AKI. The Jaffe reaction was associated with an increased odds of late AKI.
Perinatal factors that are associated with increased/decreased risk of late acute kidney injury by gestational age group. In a, the white arrow contains factors associated with reduced odds and black arrow contains factors associated with increased odds for late AKI. In b, the white Venn diagram demonstrates unique and overlapping factors within each of the gestational age cohorts associated with late AKI. The black Venn diagram (c) displays those risk factors associated with late AKI
Necrotizing enterocolitis at discharge was associated with increased odds of AKI in both the 22–28 and 29–35 week groups. Oligohydramnios, polyhydramnios and a discharge diagnosis of sepsis increased the odds of AKI in the 29–35-week group. Congenital heart disease, resuscitation with saline, nephrotoxic medications in addition to diuretics and methylxantinine exposure were associated with an increased odds of late AKI in the ≥36-week group.
Several factors were associated with a reduced odds of developing late AKI: steroids for fetal maturity and hypoglycemia (29–35 weeks), maternal hypertension and admission for intraventricular hemorrhage or seizures (≥36 weeks), and early AKI (22–28 and ≥36 weeks).
There was a significant correlation within the individual gestational age cohorts between the median serum creatinine counts and the late AKI prevalence (Fig. 4). The correlation was strongest in the 22–28 week gestational age cohort (r = 0.58, p < 0.003).
Relationship between late AKI prevalence and serum creatinine monitoring for the whole cohort and by gestational age group. Correlation between prevalence of late AKI at each individual study site and frequency of serum creatinine monitoring, represented by the median number of creatinine counts: whole group (a), 22–28 wk group (b), 29–35 wk group (c), and ≥36 wk group (d). There is a direct relationship between the number of serum creatinines and late AKI prevalence in the youngest gestational age cohort (r = 0.58, p = 0.003)
Discussion
To our knowledge, this is the first dedicated description of AKI occurring after the first week and provides evidence for the association between late neonatal AKI and poor outcomes. The uniqueness of the AWAKEN cohort is not only its large size, but that participants were enrolled across the entire gestational age spectrum, and from multiple centers and countries. Late AKI occurred in 9% of the enrolled or “higher risk” NICU population (n = 2152) and 5% total patients admitted to the NICU (n = 4270). Importantly, late AKI is independently associated with a 2.1 times higher odds of death and longer hospitalization even after accounting for multiple confounders. Understanding the risk factors and outcomes associated with late AKI can assist clinicians on both an inpatient and outpatient basis. Knowledge of these risk factors can help neonatologists identify those at high risk of AKI and allow nephrologists to distinguish those potentially at highest risk for chronic kidney disease. Finally, for pediatric hospitalists known risk factors may help detect AKI in patients transferred or readmitted from the NICU.
The prevalence of late AKI is greatest in the most preterm infants, 29% in the 22–28-week group. This finding is expected given the longer duration of hospitalization and ongoing exposures in this group. However, many centers in the AWAKEN study monitored serum creatinine infrequently in the 22–28-week group. Eleven centers had a median serum creatinine count of ≤5 following the first postnatal week Supplemental Table S6. This provides further evidence that protocols to screen for AKI should extend past the first week and be initiated during high-risk events, particularly during necrotolizing enterocolitis events in the lowest gestational age group. While not reaching statistical significance being small for gestational age was associated with a higher OR for AKI. This in combination with prematurity may be additive risks for AKI, given that being growth restricted is a risk for NEC and other morbidities associated with AKI. Importantly, the combination of growth restriction and AKI may increase risk for CKD in later childhood/adulthood.9,10
In the AWAKEN cohort, a second episode of AKI was not uncommon. In the overall group, nearly 28% (n = 56) of patients with late AKI also experienced early AKI. The timing of late AKI was approximately a week later in the group that had a prior episode of early AKI as compared to those without early AKI (median time to late AKI was 23.5 days for those with prior AKI compared to 16 days for those without prior AKI (p = 0.0002, Wilcoxon rank sums test)). Although there is a significantly increased odds of late AKI for those with a prior AKI in the crude association, it is probable that the very strong associations of late AKI with necrotizing enterocolitis, congenital heart disease, and surgical need have attenuated the association between early and late AKI in the fully adjusted models. Alternatively, the lack of association in the fully adjusted model may reflect that AKI events are discrete and independent or that late AKI was underdiagnosed due to a lack of standardization of sCr collections, particularly during high risk events. There is little known about the effect of repeated episodes of AKI in neonates. Studies in adults support the detrimental effect of recurrent AKI; both hospitalized adults or adults with diabetes who suffer recurrent AKI demonstrated a doubling of their risk for developing CKD.11,12 Unique to neonates, repeated episodes of AKI may be related to decreased available renal reserve with a GFR that does not reach adult levels until 6–12 months of age. Neonatal autopsy studies in preterm neonates show that while postnatal glomerulogenesis continues, the developing glomeruli are morphologically abnormal.13 Multiple episodes of AKI may further compromise postnatal glomerulogenesis, resulting in an even lower nephron number.14
Several maternal factors are notable for their association with a lower risk for late AKI. As with early AKI, multiple gestation was associated with a lower risk for late AKI. Conversely, the receipt of steroids, maternal hypertension, and hypoglycemia were all uniquely associated with a decreased risk for late AKI. Each of these factors represents a setting in which a mother might receive exogenous or endogenous increase in steroids to mature the lungs which may also inturn mature the kidneys.
As perinatal events and interventions determine many of the early AKI risk factors, we speculated that there would be unique factors that discriminated those with late AKI. Several unique perinatal factors were associated with late onset AKI including intubation, oligohydramnios, polyhydramnios and mild-moderate renal anomalies. Late AKI was associated with resuscitation efforts with intubation, potentially highlighting a sicker population of infants. Improvements in perinatal strategies, particularly with the trend toward a trial of early continuous positive airway pressure in lieu of intubation may extend beneficial effects including reduction of AKI.15,16,17,18 The presence of oligohydramnios and polyhydramnios were both associated with an increased risk of late AKI. Oligohydramnios may be associated with fetal renal anomalies/dysfunction affecting lung development (potentially necessitating intubation), and polyhydramnios may be associated with gastrointestinal abnormalities that impair swallowing of amniotic fluid. The finding of increased risk of late AKI in the presence of alterations in amniotic fluid volume therefore may be a proxy for fetal conditions such as renal or gastrointestinal abnormalities.
Specific associations between gastrointestinal diseases such as necrotizing enterocolitis and late AKI were detected in this study. The suspicion of necrotizing enterocolitis on admission in the 22–28-week group and discharged with NEC as diagnosis in the 22–35-week group were both associated with an increased risk of late AKI. A recent single-center study of neonates with an average gestational age of 30.8 weeks demonstrated a strong association between necrotizing enterocolitis and AKI.19 Animal models of necrotizing enterocolitis show not only intestinal inflammation, but also a robust kidney inflammatory response with infiltration of mononuclear cells and disruption of the tight junctions within the kidney.20 These findings suggest that the mechanism for AKI following necrotizing enterocolitis may not solely be related to hypoperfusion of the kidney during sepsis, but also to the resultant inflammatory response. Care bundles and exclusive use of human milk feedings along with probiotics aimed at preventing necrotizing enterocolitis may decrease the incidence of late AKI and other associated morbidities.
Several admission and discharge diagnoses were associated with a risk of late AKI including congenital heart disease, surgical need, necrotizing enterocolitis, sepsis, and patent ductus arteriosus. Previous reports have indicated an increased risk of developing AKI after surgical repair of congenital heart disease.21,22 Similarly, we observed a 10-fold increase in the risk for developing late AKI in neonates with congenital heart disease. Because of the retrospective nature of our data, we are unable to differentiate between AKI development pre- or post-operatively. Similarly, the AWAKEN dataset did not contain information on the temporal relationship of late AKI and discharge diagnoses such as necrotizing enterocolitis, and sepsis. The postnatal care of infants with surgical diagnoses, cardiac anomalies, and necrotizing enterocolitis may need to be further refined, with particular attention paid to fluid management, practice guidelines for the early and routine monitoring of serial creatinine and glomerular filtration rate measurements, and early involvement of nephrology in the management of AKI.
A number of medications were associated with increased risk of late AKI including diuretics (fully adjusted OR 2.38), vasopressors (fully adjusted OR 2.42) and NSAID’s (fully adjusted OR 2.68). This demonstrates the importance of attention to medications and their potential cumulative effects, supporting programs such as NINJA23 to minimize those risks. The cumulative exposure of nephrotoxins, including NSAIDs, may influence the development of late AKI. This is supported by the animal data that shows prenatal exposure to indomethacin results in reduce nephron number24 and an increase of urinary podocytes in indomethacin-treated neonates.25
Despite the strengths of this study, AWAKEN has several limitations. One important limitation is the variability in the method and the frequency of creatinine measurements between institutions. We have demonstrated that late AKI prevalence correlates with the number of creatinines measured in the 22–28-week group; therefore, kidney injury may have been under-reported due to infrequent serum creatinine monitoring (Fig. 4). Of the 2152 enrolled, 608 participates had <2 creatinine measurements and were classified as no AKI based on sensitivity analysis. However, the risk for bias is prominent and this study therefore highlights the need to standardize monitoring practices in the NICU. These renal screening protocols should be based on specific risk factors providing meaningful information for the most ‘at risk’ populations, while limiting cost and negative effects of blood draws in those who have low likelihood to develop AKI. The increased risk of AKI as determined by method of analysis is important to note—as it may denote potential bias or a true increased risk. To better understand the validity of this relationship, we examined the fully adjusted model in Table 5 stratified by Jaffe and enzymatic reactions. There was no difference in the estimated associations between the two reaction types; thus, we believe any bias through the use of Jaffe would be non-differential and our reported associations are likely underestimates of the true associations. The Jaffe methodology for assessment of creatinine can be affected by bilirubin or other pigmented influences. The different analysis methods and automated analysers produce higher levels of creatinine with the Jaffe method, but this is predominantly at low levels of creatinine.26,27 If the definition of AKI was based on a serum creatinine cut off, the association between late AKI and the Jaffe reaction would be more clear. However the KDIGO definition is based on changes from a baseline, making it important for future prospective studies to take into account laboratory methods for creatinine assessment to fully answer this question. Other limitations of the AWAKEN study are inherent to those related to a retrospective analysis. Data were extracted from all individual institutions electronic medical record or the subject’s paper chart. The sources for bias included misclassification bias because there was a potentially greater risk for missing a true AKI event in those participants with fewer measured serum creatinines. Importantly, this analysis did not evaluate all neonates or even all neonates in the NICU, only those who met inclusion criteria. The indications for a number of interventions or therapies were unavailable, the temporal relationship between discharge diagnoses and AKI could not be assessed and our findings for neonates with congenital heart disease are not generalizable as only those with surgery performed >7 days after birth were included. These details are important in designing future prospective studies as data extraction from the subject’s chart might have inherent accuracy issues.28
In conclusion, late AKI is independently associated with a 2.1 times odds of death and longer hospitalization with worse outcomes when stratified by severity of AKI. The prevalence of AKI may be even higher due to the variability in frequency of creatinine measurements, and is an important factor for care providers to consider in caring for neonates in the NICU, as well as in planning future research. Care providers should be aware that in addition to a lower gestation age and Apgar scores, intubation, polyhydramnios, oligohydramnios, any renal anomalies, congenital heart disease, surgical need, necrotizing enterocolitis, sepsis, patient ductus arteriosus, and urinary tract infections are all independently associated with late AKI. Importantly, nearly one third of neonates with late AKI had an episode of early AKI, potentially increasing the risk for long-term chronic kidney disease. Although it is challenging to study the long-term kidney impact of AKI, research in this area should be a priority given the prevalence of AKI and potential consequence of CKD.
References
Jetton, J. G. et al. Assessment of worldwide acute kidney injury epidemiology in neonates: design of a retrospective cohort study. Front. Pediatr. 4, 68 (2016).
Jetton, J. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Charlton, J. R. et al. on behalf of the Neonatal Kidney Collaborative. Incidence and Risk Factors of Early Onset Neonatal Acute Kidney Injury. CJASN. (2018).
Carmody, J. B., Swanson, J. R., Rhone, E. T., Charlton, J. R. Recognition and Reporting of Acute Kidney Injury in Very Low Birth Weight Infants. Clin. J. Am. Soc. Nephrol. 9, 2036–2043 (2014). PMID: 25280497. PMCID: 4255405.
Askenazi, D. J. et al. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr. Nephrol. 28, 661–666 (2013).
Jetton, J. G. & Askenazi, D. J. Acute kidney injury in the neonate. Clin. Perinatol. 41, 487–502 (2014).
Jacobs, S. E., et al Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev.:CD003311 (2013).
Kellum, J. A., Lameire, N. & KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit. Care 17, 204 (2013).
Hsu, C. W., Yamamoto, K. T., Henry, R. K., De Roos, A. J. & Flynn, J. T. Prenatal risk factors for childhood CKD. J. Am. Soc. Nephrol. 25, 2105–11 (2014).
White, S. L. et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am. J. Kidney Dis. 54, 248–261 (2009).
Rodriguez, E. et al. Impact of recurrent acute kidney injury on patient outcomes. Kidney Blood. Press. Res. 43, 34–44 (2018).
Thakar, C. V., Christianson, A., Himmelfarb, J. & Leonard, A. C. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin. J. Am. Soc. Nephrol. 6, 2567–2572 (2011).
Sutherland, M. R. et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J. Am. Soc. Nephrol. 22, 1365–1374 (2011).
Rodriguez, M. M. et al. Comparative renal histomorphometry: a case study of oligonephropathy of prematurity. Pediatr. Nephrol. 20, 945–949 (2005).
Pfister, R. H. & Soll, R. F. Initial respiratory support of preterm infants: the role of CPAP, the INSURE method, and noninvasive ventilation. Clin. Perinatol. 39, 459–481 (2012).
Tapia, J. L. et al. Randomized trial of early bubble continuous positive airway pressure for very low birth weight infants. J. Pediatr. 161, 75–80.e1 (2012).
Morley, C. J. et al. Nasal CPAP or intubation at birth for very preterm infants. N. Engl. J. Med. 358, 700–708 (2008).
SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Finer, N. N. et al. Early CPAP versus surfactant in extremely preterm infants. N. Engl. J. Med. 362, 1970–1979 (2010).
Criss, C. N., et al. Acute kidney injury in necrotizing enterocolitis predicts mortality. Pediatr. Nephrol. 33, 503–510 (2017). Epub 2017 Oct 5.
Garg, P. M., Tatum, R., Ravisankar, S., Shekhawat, P. S. & Chen, Y. H. Necrotizing enterocolitis in a mouse model leads to widespread renal inflammation, acute kidney injury, and disruption of renal tight junction proteins. Pediatr. Res. 78, 527–532 (2015).
Morgan, C. J. et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J. Pediatr. 162, 120–127.e1 (2013).
Blinder, J. J. et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J. Thorac. Cardiovasc. Surg. 143, 368–374 (2012).
Goldstein, S. L. et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 90, 212–221 (2016).
Kent, A. L. et al. Indomethacin administered early in the postnatal period results in reduced glomerular number in the adult rat. Am. J. Physiol. Ren. Physiol. 307, F1105–F1110 (2014).
Kent, A. L., Brown, L., Broom, M., Broomfield, A. & Dahlstrom, J. E. Increased urinary podocytes following indomethacin suggests drug-induced glomerular injury. Pediatr. Nephrol. 27, 1111–1117 (2012).
Kume, T., Saglam, B., Ergon, C. & Sisman, A. R. Evaluation and comparison of Abbott Jaffe and enzymatic creatinine methods: could the old method meet the new requirements? J. Clin. Lab. Anal. 32 (2018) https://doi.org/10.1002/jcla.22168.
Hermida, F. J. et al. Comparison between ADVIA Chemistry systems Enzymatic Creatinine_2 method and ADVIA chemistry systems creatinine method (kinetic Jaffe method) for determining creatinine. Scand. J. Clin. Lab. Invest. 74, 629–636 (2014).
Stausberg, J., Koch, D., Ingenerf, J. & Betzler, M. Comparing paper-based with electronic patient records: lessons learned during a study on diagnosis and procedure codes. J. Am. Med. Inform. Assoc. 10, 470–477 (2003).
Acknowledgments
Cincinnati Children’s Hospital Center for Acute Care Nephrology provided funding to create and maintain the AWAKEN Medidata Rave electronic database. The Pediatric and Infant Center for Acute Nephrology (PICAN) provided support for web meetings, for the NKC steering committee annual meeting at the University of Alabama at Birmingham (UAB), as well as support for some of the AWAKEN investigators at UAB (DA, LBJ, RJG). PICAN is part of the Department of Pediatrics at the University of Alabama at Birmingham (UAB), and is funded by Children’s of Alabama Hospital, the Department of Pediatrics, UAB School of Medicine, and UAB’s Center for Clinical and Translational Sciences (CCTS, NIH grant UL1TR001417). Finally, the AWAKEN study was supported at the University of New Mexico by the Clinical and Translational Science Center (CTSC, NIH grant UL1TR001449). The authors would also like to thank the outstanding work of the following clinical research personnel and colleagues for their involvement in AWAKEN: Ariana Aimani, Samantha Kronish, Ana Palijan, MD, Michael Pizzi—Montreal Children's Hospital, McGill University Health Centre, Montreal, Quebec, Canada; Laila Ajour, BS, Julia Wrona, BS—University of Colorado, Children's Hospital Colorado, Aurora, Colorado, USA; Melissa Bowman, RN—University of Rochester, Rochester, New York, USA; Teresa Cano, RN, Marta G. Galarza, MD, Wendy Glaberson, MD, Aura Arenas Morales, MD, Denisse Cristina Pareja Valarezo, MD—Holtz Children’s Hospital, University of Miami, Miami, Florida, USA; Sarah Cashman, BS, Madeleine Stead, BS—University of Iowa Children’s Hospital, Iowa City, Iowa, USA; Jonathan Davis, MD, Julie Nicoletta, MD—Floating Hospital for Children at Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts, USA; Alanna DeMello—British Columbia Children’s Hospital, Vancouver, British Columbia, Canada; Lynn Dill, RN—University of Alabama at Birmingham, Birmingham, Alabama, USA; Ellen Guthrie, RN—MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio, USA, Nicholas L. Harris, BS, Susan M. Hieber, MSQM—C.S. Mott Children's Hospital, University of Michigan, Ann Arbor, Michigan, USA; Katherine Huang, Rosa Waters—University of Virginia Children’s Hospital, Charlottesville, Virginia, USA; Judd Jacobs, Ryan Knox, BS, Hilary Pitner, MS, Tara Terrell—Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; Nilima Jawale, MD—Maimonides Medical Center, Brooklyn, New York, USA; Emily Kane—Australian National University, Canberra, Australia; Vijay Kher, DM, Puneet Sodhi, MBBS—Medanta Kidney Institute, The Medicity Hospital, Gurgaon, Haryana, India; Grace Mele—New York College of Osteopathic Medicine, Westbury, New York, USA; Patricia Mele, DNP—Stony Brook Children’s Hospital, Stony Brook, New York, USA; Charity Njoku, Tennille Paulsen, Sadia Zubair—Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas, USA; Emily Pao—University of Washington, Seattle Children's Hospital, Seattle, Washington, USA; Becky Selman RN, Michele Spear, CCRC—University of New Mexico Health Sciences Center Albuquerque, New Mexico, USA; Melissa Vega, PA-C—The Children’s Hospital at Montefiore, Bronx, New York, USA; Leslie Walther RN—Washington University, St. Louis, Missouri, USA.
The Neonatal Kidney Collaborative (NKC) members listed in Appendix are non-author contributors and served as collaborators and site investigators for the AWAKEN study. They collaborated in protocol development and review, local IRB submission, data collection and participated in drafting or review of the manuscript. The Neonatal Kidney Collaborative (NKC) members listed below are non-author contributors and served as collaborators and site investigators for the AWAKEN study and deserve a PUBMED citation. They collaborated in protocol development and review, local IRB submission, data collection and participated in drafting or review of the manuscript.
Neonatal Kidney Collaborative (NKC)
Namasivayam Ambalavanan, MD13, David T. Selewski, MD12, Jeffery Fletcher, PhD14, Carolyn L Abitbol, MD15, Marissa DeFreitas, MD15, Shahnaz Duara, MD15, Ronnie Guillet, MD, PhD16, Erin Rademacher, MD16, Carl D’Angio, MD16, Maroun J. Mhanna, MD17, Rupesh Raina, MD17, Deepak Kumar, MD17, Ayse Akcan Arikan, MD10, Stuart L. Goldstein, MD18, Amy T. Nathan, MD18, Juan C. Kupferman, MD19, Alok Bhutada, MD19, Elizabeth Bonachea, MD20, John Mahan, MD20, Arwa Nada, MBBCH21, Jennifer Jetton, MD22, Tarah T. Colaizy, MD22, Jonathan M. Klein, MD22, F. Sessions Cole, MD23, T. Keefe Davis, MD23, Lawrence Milner, MD24, Alexandra Smith, MD24, Kimberly Reidy, MD25, Frederick J. Kaskel, MD25, Katja M. Gist, DO5, Mina H. Hanna, MD26, Craig S. Wong, MD27, Catherine Joseph, MD27, Tara DuPont, MD27, Amy Staples, MD27, Surender Khokhar, MD28, Sofia Perazzo, MD11, Patricio E. Ray, MD11, Cherry Mammen, MD29, Anne Synnes, MDCM29, Pia Wintermark, MD30, Sidharth K. Sethi, MD31, Sanjay Wazir, MD32, Smriti Rohatgi, MD33, Danielle E. Soranno, MD5, Katja M. Gist, DO5, Aftab S. Chishti, MD26, Mina H. Hanna, MD26, Robert Woroniecki, MD34, Shanty Sridhar, MD34, Jonathan R. Swanson MD35, Michael Zappitelli, MD36
Author contributions
J.R.C. and A.L.K. contributed to the conceptualization and design of the study, collected data, aided the data analysis, and drafted the initial manuscript. L.B. and R.G. completed the data analysis and interpretation, contributed to the drafting and revising the manuscript. D.A. contributed to the conceptualization and design of the study, aided in data analysis, and contributed to the revising of the manuscript for critically important intellectual content. P.D.B., M.F., J.G., S.H., S.I., A.M., R.K.O., S.R., C.J.R., M.R., S.S., and M.S. contributed to the study design, collecting or supervising the collection of data, and revising the manuscript for critically important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
J.R.C. is a co-owner of Sindri Technologies, LLC. She receives funding from the National Institutes of Health-National Institutes of Diabetes and Digestive and Kidney Diseases (R01DK110622, R01DK111861). D.J.A. serves on the speaker board for Baxter (Baxter, USA), and the Acute Kidney Injury (AKI) Foundation (Cincinnati, OH, USA); he also receives grant funding for studies not related to this manuscript from Octapharma AG (Switzerland), and the National Institutes of Health—National Institutes of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK, R01 DK103608). S.H. is also funded through NIH-NIDDK, R01 DK103608. All the remaining authors declare no competing interests.
Clinical Trial registry name and registration number
Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates (AWAKEN), NCT02443389.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical Trial registry name and registration number: Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates (AWAKEN), NCT02443389
Appendix
NKC Contributors
Namasivayam Ambalavanan, MD—Department of Pediatrics, University of Alabama at Birmingham, Birmingham, AL, USA.
David T. Selewski, MD—C.S. Mott Children's Hospital, University of Michigan, Ann Arbor, MI, USA.
Jeffery Fletcher, PhD—Centenary Hospital for Women and Children, Canberra Hospital, Australian National University Medical School, Canberra, Australia.
Carolyn L Abitbol, MD, Marissa DeFreitas, MD, Shahnaz Duara, MD—Holtz Children's Hospital, University of Miami, Miami, FL, USA.
Ronnie Guillet, MD, PhD, Erin Rademacher, MD, Carl D’Angio, MD—Golisano Children’s Hospital, University of Rochester, Rochester, NY, USA.
Maroun J. Mhanna, MD, Rupesh Raina, MD, Deepak Kumar, MD—MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio, USA.
Ayse Akcan Arikan, MD—Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas, USA.
Stuart L. Goldstein, MD, Amy T. Nathan, MD—Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Juan C. Kupferman, MD, Alok Bhutada, MD—Maimonides Medical Center, Brooklyn, New York, USA.
Elizabeth Bonachea, MD, John Mahan, MD—Nationwide Children’s Hospital, Columbus, Ohio, USA.
Arwa Nada, MBBCH—LeBoneur Children’s Hospital, University of Tennessee Health Science Center, Memphis TN, USA
Jennifer Jetton, MD, Tarah T. Colaizy, MD, Jonathan M. Klein, MD—University of Iowa Children’s Hospital, Iowa City, Iowa, USA.
F. Sessions Cole, MD, T. Keefe Davis, MD—Washington University, St. Louis, Missouri, USA.
Lawrence Milner, MD, Alexandra Smith, MD—Tufts University School of Medicine, Boston, Massachusetts, USA.
Kimberly Reidy, MD, Frederick J. Kaskel, MD—The Children’s Hospital at Montefiore, Bronx, New York, USA
Katja M. Gist, DO—University of Colorado, Children's Hospital Colorado, Aurora, Colorado, USA.
Mina H. Hanna, MD—University of Kentucky, Lexington, Kentucky, USA.
Craig S. Wong, MD, Catherine Joseph, MD, Tara DuPont, MD, Amy Staples, MD—University of New Mexico Health Sciences Center, Albuquerque, New Mexico, USA.
Surender Khokhar, MD—Apollo Cradle, Gurgaon, Haryana, India.
Sofia Perazzo, MD, Patricio E. Ray, MD— Children’s National Medical Center, George Washington University School of Medicine and the Health Sciences, Washington DC, USA.
Cherry Mammen, MD, Anne Synnes, MDCM—British Columbia Children's Hospital, Vancouver, British Columbia, Canada.
Pia Wintermark, MD— Montreal Children's Hospital, McGill University Health Centre, Montreal, Quebec, Canada
Sidharth K. Sethi, MD—Kidney and Urology Institute. Medanta The Medicity, Gurgaon, India
Sanjay Wazir, MD—Neonatology, Cloudnine Hospital, Gurgaon, Haryana, India
Smriti Rohatgi, MD—Medanta, The Medicity, Gurgaon, Haryana, India
Danielle E. Soranno, MD, Katja M. Gist, DO—University of Colorado, Children's Hospital Colorado, Aurora, Colorado, USA.
Aftab S. Chishti, MD, Mina H. Hanna, MD—University of Kentucky, Lexington, Kentucky, USA.
Robert Woroniecki, MD, Shanty Sridhar, MD—Stony Brook School of Medicine, Stony Brook, NY, USA
Jonathan R. Swanson, MD—University of Virginia Children’s Hospital, Charlottesville, Virginia, USA
Michael Zappitelli, MD—Toronto Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
Supplementary information
Rights and permissions
About this article
Cite this article
Charlton, J.R., Boohaker, L., Askenazi, D. et al. Late onset neonatal acute kidney injury: results from the AWAKEN Study. Pediatr Res 85, 339–348 (2019). https://doi.org/10.1038/s41390-018-0255-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0255-x
This article is cited by
-
Perinatal risk factors associated with acute kidney injury severity and duration among infants born extremely preterm
Pediatric Research (2024)
-
Pharmacovigilance of nephrotoxic drugs in neonates: the Pottel method for acute kidney injury detection in ELBW neonates
Pediatric Nephrology (2024)
-
Receipt of high-frequency ventilation is associated with acute kidney injury in very preterm neonates
Pediatric Nephrology (2024)
-
Acute kidney injury in infants diagnosed with congenital diaphragmatic hernia
Pediatric Research (2023)
-
Preceding risks and mortality outcomes of different neonatal acute kidney injury in preterm infants
Pediatric Research (2023)