Abstract
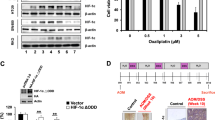
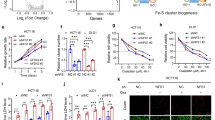
Although the role of isocitrate dehydrogenase (IDH) mutation in promoting cancer development has been well-characterized, the impact of wild-type IDH on cancer cells remains unclear. Here we show that the wild-type isocitrate dehydrogenase 2 (IDH2) is highly expressed in colorectal cancer (CRC) cells, and plays an unexpected role in protecting the cancer cells from oxidative damage. Genetic abrogation of IDH2 in CRC cells leads to reactive oxygen species (ROS)-mediated DNA damage and an accumulation of 8-oxoguanine with DNA strand breaks, which activates DNA damage response (DDR) with elevated γH2AX and phosphorylation of ataxia telangiectasia-mutated (ATM) protein, leading to a partial cell cycle arrest and eventually cell senescence. Mechanistically, the suppression of IDH2 results in a reduction of the tricarboxylic acid (TCA) cycle activity due to a decrease in the conversion of isocitrate to α-ketoglutarate (α-KG) with a concurrent decrease in NADPH production, leading to ROS accumulation and oxidative DNA damage. Importantly, abrogation of IDH2 inhibits CRC cell growth in vitro and in vivo, and renders CRC cells more vulnerable to DNA-damaging drugs. Screening of an FDA-approved drug library has identified oxaliplatin as a compound highly effective against CRC cells when IDH2 was suppressed. Our study has uncovered an important role of the wild-type IDH2 in protecting DNA from oxidative damage, and provides a novel biochemical basis for developing metabolic intervention strategy for cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated or analyzed for the current study are available from the corresponding author on reasonable request.
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell.2011;144:646–74.
Dang L, Su SM. Isocitrate dehydrogenase mutation and (R)−2-hydroxyglutarate: from basic discovery to therapeutics development. Annu Rev Biochem. 2017;86:305–31.
Yan H, Bigner DD, Velculescu V, Parsons DW. Mutant metabolic enzymes are at the origin of gliomas. Cancer Res. 2009;69:9157–9.
King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene.2006;25:4675–82.
Yen KE, Bittinger MA, Su SM, Fantin VR. Cancer-associated IDH mutations: biomarker and therapeutic opportunities. Oncogene.2010;29:6409–17.
Dalziel K. Isocitrate dehydrogenase and related oxidative decarboxylases. FEBS Lett. 1980;117:K45–55.
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature.2009;462:739–44.
Yang H, Ye D, Guan KL, Xiong Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res. 2012;18:5562–71.
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N. Engl J Med. 2009;360:765–73.
Montalban-Bravo G, DiNardo CD. The role of IDH mutations in acute myeloid leukemia. Future Oncol. 2018;14:979–93.
Waitkus MS, Diplas BH, Yan H. Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell. 2018;34:186–95.
McBrayer SK, Mayers JR, DiNatale GJ, Shi DD, Khanal J, Chakraborty AA, et al. Transaminase inhibition by 2-hydroxyglutarate impairs glutamate biosynthesis and redox homeostasis in Glioma. Cell.2018;175:101–.e125.
Ježek P. 2-Hydroxyglutarate in cancer cells. Antioxid Redox Signal. 2020;33:903–26.
Short NJ, Konopleva M, Kadia TM, Borthakur G, Ravandi F, DiNardo CD, et al. Advances in the treatment of acute myeloid. Leukemia: N. Drugs N. Chall Cancer Discov. 2020;10:506–25.
Molenaar RJ, Maciejewski JP, Wilmink JW, van Noorden CJF. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene.2018;37:1949–60.
Bergaggio E, Piva R. Wild-type IDH enzymes as actionable targets for cancer therapy. Cancers (Basel). 2019;11:563.
Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics.2004;84:1014–20.
Li J, He Y, Tan Z, Lu J, Li L, Song X, et al. Wild-type IDH2 promotes the Warburg effect and tumor growth through HIF1α in lung cancer. Theranostics.2018;8:4050–61.
Liu WR, Tian MX, Jin L, Yang LX, Ding ZB, Shen YH, et al. High expression of 5-hydroxymethylcytosine and isocitrate dehydrogenase 2 is associated with favorable prognosis after curative resection of hepatocellular carcinoma. J Exp Clin Cancer Res. 2014;33:32.
Tian GY, Zang SF, Wang L, Luo Y, Shi JP, Lou GQ. Isocitrate dehydrogenase 2 suppresses the invasion of hepatocellular carcinoma cells via matrix metalloproteinase 9. Cell Physiol Biochem. 2015;37:2405–14.
Wu D. Isocitrate dehydrogenase 2 inhibits gastric cancer cell invasion via matrix metalloproteinase 7. Tumour Biol. 2016;37:5225–30.
Zhou L, Wang F, Sun R, Chen X, Zhang M, Xu Q, et al. SIRT5 promotes IDH2 desuccinylation and G6PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep. 2016;17:811–22.
Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science.2006;314:268–74.
Lv Q, Xing S, Li Z, Li J, Gong P, Xu X, et al. Altered expression levels of IDH2 are involved in the development of colon cancer. Exp Ther Med. 2012;4:801–6.
Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9.
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017; 67:177–93.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51.
Wolvetang EJ, Johnson KL, Krauer K, Ralph SJ, Linnane AW. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994;339:40–4.
Neuzil J, Wang XF, Dong LF, Low P, Ralph SJ. Molecular mechanism of ‘mitocan’-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett. 2006;580:5125–9.
Sabharwal S, Schumacker P. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–21.
Klaunig J. Oxidative stress and cancer. Curr Pharm Des. 2018;24:4771–8.
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis .2007;12:913–22.
Yang Y, Karakhanova S, Hartwig W, D’Haese JG, Philippov PP, Werner J, et al. Mitochondria and mitochondrial ROS in cancer: novel targets for anticancer therapy. J Cell Physiol. 2016;231:2570–81.
Gorrini C, Harris I, Mak T. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–47.
Luo J, Robinson JP, Shi R. Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochem Int. 2005;47:449–57.
Cha H, Lee S, Hwan Kim S, Kim H, Lee DS, Lee HS, et al. Increased susceptibility of IDH2-deficient mice to dextran sodium sulfate-induced colitis. Redox Biol. 2017;13:32–8.
Molenaar RJ, Botman D, Smits MA, Hira VV, van Lith SA, Stap J, et al. Radioprotection of IDH1-mutated cancer cells by the IDH1-mutant inhibitor AGI-5198. Cancer Res. 2015;75:4790–802.
Salehi F, Behboudi H, Kavoosi G, Ardestani SK. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: a comparison of the biological characteristics of citrus pectin and apple pectin. Sci Rep. 2018;8:13902.
Molenaar RJ, Radivoyevitch T, Nagata Y, Khurshed M, Przychodzen B, Makishima H, et al. IDH1/2 mutations sensitize acute myeloid leukemia to PARP inhibition and this is reversed by IDH1/2-mutant inhibitors. Clin Cancer Res. 2018;24:1705–15.
Matt S, Hofmann TG. The DNA damage-induced cell death response: a roadmap to kill cancer cells. Cell Mol Life Sci. 2016;73:2829–50.
Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91.
Sulkowski PL, Corso CD, Robinson ND, Scanlon SE, Purshouse KR, Bai H, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med. 2017;9:aal2463.
Kong MJ, Han SJ, Kim JI, Park JW, Park KM. Mitochondrial NADP(+)-dependent isocitrate dehydrogenase deficiency increases cisplatin-induced oxidative damage in the kidney tubule cells. Cell Death Dis. 2018;9:488.
Torrens-Mas M, Pons DG, Sastre-Serra J, Oliver J, Roca P. SIRT3 silencing sensitizes breast cancer cells to cytotoxic treatments through an increment in ROS production. J Cell Biochem. 2017;118:397–406.
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9.
Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia.2007;9:166–80.
Acknowledgements
We would like to thank Yanyu Zhang, Mingquan Zhang and Bing-ling Luo for technical assistance, Haijing Deng and Dr. Hui Zhang for helpful discussions. Some of the cartoons in this article were created using the drawing tools provided by BioRender (https://biorender.com). This work was support in part by grants from the National Key R&D Program of China (2020YFA0803300, 2018YFC0910203), and from the National Natural Science Foundation of China (81872440, 81672952).
Author information
Authors and Affiliations
Contributions
SQ designed and performed research, analyzed data, and drafted paper. WL performed animal experiments. CG, PZ, NM, and HZ participated in bioinformatics analysis and biochemical/cellular experiments. JL participated in research design and paper writing. SW and PH designed the supervised the research. All authors have read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Qiao, S., Lu, W., Glorieux, C. et al. Wild-type IDH2 protects nuclear DNA from oxidative damage and is a potential therapeutic target in colorectal cancer. Oncogene 40, 5880–5892 (2021). https://doi.org/10.1038/s41388-021-01968-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01968-2
This article is cited by
-
SIRT5-mediated ME2 desuccinylation promotes cancer growth by enhancing mitochondrial respiration
Cell Death & Differentiation (2024)
-
Metabolic reprogramming in colorectal cancer: regulatory networks and therapy
Cell & Bioscience (2023)
-
IDH2, a novel target of OGT, facilitates glucose uptake and cellular bioenergy production via NF-κB signaling to promote colorectal cancer progression
Cellular Oncology (2023)
-
Genetic mutations affecting mitochondrial function in cancer drug resistance
Genes & Genomics (2023)
-
Wild-type IDH1 Knockout Leads to G0/G1 Arrest, Impairs Cancer Cell Proliferation, Altering Glycolysis, and the TCA Cycle in Colon Cancer
Biochemical Genetics (2023)