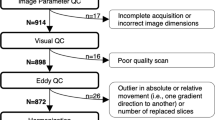
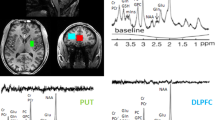
Abstract
Seven Tesla magnetic resonance spectroscopy (7T MRS) offers a precise measurement of metabolic levels in the human brain via a non-invasive approach. Studying longitudinal changes in brain metabolites could help evaluate the characteristics of disease over time. This approach may also shed light on how the age of study participants and duration of illness may influence these metabolites. This study used 7T MRS to investigate longitudinal patterns of brain metabolites in young adulthood in both healthy controls and patients. A four-year longitudinal cohort with 38 patients with first episode psychosis (onset within 2 years) and 48 healthy controls was used to examine 10 brain metabolites in 5 brain regions associated with the pathophysiology of psychosis in a comprehensive manner. Both patients and controls were found to have significant longitudinal reductions in glutamate in the anterior cingulate cortex (ACC). Only patients were found to have a significant decrease over time in γ-aminobutyric acid, N-acetyl aspartate, myo-inositol, total choline, and total creatine in the ACC. Together we highlight the ACC with dynamic changes in several metabolites in early-stage psychosis, in contrast to the other 4 brain regions that also are known to play roles in psychosis. Meanwhile, glutathione was uniquely found to have a near zero annual percentage change in both patients and controls in all 5 brain regions during a four-year follow-up in young adulthood. Given that a reduction of the glutathione in the ACC has been reported as a feature of treatment-refractory psychosis, this observation further supports the potential of glutathione as a biomarker for this subset of patients with psychosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014;39:1–36.
Poels EMP, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi-Dargham A, et al. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophr Res. 2014;152:325–32.
Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–5.
Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61:1279–85.
Pradhan S, Bonekamp S, Gillen JS, Rowland LM, Wijtenburg SA, Edden RAE, et al. Comparison of single voxel brain MRS AT 3T and 7T using 32-channel head coils. Magn Reson Imaging. 2015;33:1013–8.
Tkác I, Oz G, Adriany G, Uğurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62:868–79.
Brandt AS, Unschuld PG, Pradhan S, Lim IAL, Churchill G, Harris AD, et al. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: A (1)H MRS Study at 7 Tesla. Schizophr Res. 2016;172:101–5.
Reid MA, Salibi N, White DM, Gawne TJ, Denney TS, Lahti AC. 7T proton magnetic resonance spectroscopy of the anterior cingulate cortex in first-episode schizophrenia. Schizophr Bull. 2019;45:180–9.
Kumar J, Liddle EB, Fernandes CC, Palaniyappan L, Hall EL, Robson SE, et al. Glutathione and glutamate in schizophrenia: a 7T MRS study. Mol Psychiatry. 2020;25:873–82.
Thakkar KN, Rösler L, Wijnen JP, Boer VO, Klomp DWJ, Cahn W, et al. 7T proton magnetic resonance spectroscopy of gamma-aminobutyric acid, glutamate, and glutamine reveals altered concentrations in patients with schizophrenia and healthy siblings. Biol Psychiatry. 2017;81:525–35.
Dempster K, Jeon P, MacKinley M, Williamson P, Théberge J, Palaniyappan L. Early treatment response in first episode psychosis: a 7-T magnetic resonance spectroscopic study of glutathione and glutamate. Mol Psychiatry. 2020;25:1640–50.
Rowland LM, Pradhan S, Korenic S, Wijtenburg SA, Hong LE, Edden RA, et al. Elevated brain lactate in schizophrenia: a 7T magnetic resonance spectroscopy study. Transl Psychiatry. 2016;6:e967.
Marsman A, Mandl RCW, Klomp DWJ, Bohlken MM, Boer VO, Andreychenko A, et al. GABA and glutamate in schizophrenia: a 7 T 1H-MRS study. Neuroimage Clin. 2014;6:398–407.
Godlewska BR, Minichino A, Emir U, Angelescu I, Lennox B, Micunovic M, et al. Brain glutamate concentration in men with early psychosis: a magnetic resonance spectroscopy case-control study at 7T. Transl Psychiatry. 2021;11:367.
Jeon P, Limongi R, Ford SD, Mackinley M, Dempster K, Théberge J, et al. Progressive changes in glutamate concentration in early stages of schizophrenia: a longitudinal 7-Tesla MRS study. Schizophrenia Bull Open. 2021;2:sgaa072.
Wang AM, Pradhan S, Coughlin JM, Trivedi A, DuBois SL, Crawford JL, et al. Assessing brain metabolism With 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiatry. 2019;76:314–23.
Bustillo JR, Mayer EG, Upston J, Jones T, Garcia C, Sheriff S, et al. Increased glutamate plus glutamine in the right middle cingulate in early schizophrenia but not in bipolar psychosis: a whole brain 1H-MRS study. Front Psychiatry. 2021;12:660850.
Merritt K, McGuire PK, Egerton A, 1H-MRS in Schizophrenia Investigators, Aleman A, Block W, et al. Association of age, antipsychotic medication, and symptom severity in schizophrenia with proton magnetic resonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psychiatry. 2021;78:667–81.
McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19:15–33.
Egerton A, Modinos G, Ferrera D, McGuire P. Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl Psychiatry. 2017;7:e1147.
Marenco S, Meyer C, Kuo S, van der Veen JW, Shen J, DeJong K, et al. Prefrontal GABA levels measured with magnetic resonance spectroscopy in patients with psychosis and unaffected siblings. Am J Psychiatry. 2016;173:527–34.
Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–104.
Rowland LM, Summerfelt A, Wijtenburg SA, Du X, Chiappelli JJ, Krishna N, et al. Frontal glutamate and γ-aminobutyric acid levels and their associations with mismatch negativity and digit sequencing task performance in schizophrenia. JAMA Psychiatry. 2016;73:166–74.
Bojesen KB, Ebdrup BH, Jessen K, Sigvard A, Tangmose K, Edden RAE, et al. Treatment response after 6 and 26 weeks is related to baseline glutamate and GABA levels in antipsychotic-naïve patients with psychosis. Psychol Med. 2020;50:2182–93.
Dixon BJ, Kumar J, Danielmeier C. Frontal neural metabolite changes in schizophrenia and their association with cognitive control: a systematic review. Neurosci Biobehav Rev. 2022;132:224–47.
Egerton A, Broberg BV, Van Haren N, Merritt K, Barker GJ, Lythgoe DJ, et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre 1H-MRS study (OPTiMiSE). Mol Psychiatry. 2018;23:2145–55.
de la Fuente-Sandoval C, León-Ortiz P, Azcárraga M, Stephano S, Favila R, Díaz-Galvis L, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. 2013;70:1057–66.
de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, León-Ortiz P, Rodríguez-Mayoral O, Jung-Cook H, et al. Prefrontal and striatal gamma-aminobutyric acid levels and the effect of antipsychotic treatment in first-episode psychosis patients. Biol Psychiatry. 2018;83:475–83.
Marsman A, van den Heuvel MP, Klomp DWJ, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophr Bull. 2013;39:120–9.
Théberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–34.
Goff DC, Hennen J, Lyoo IK, Tsai G, Wald LL, Evins AE, et al. Modulation of brain and serum glutamatergic concentrations following a switch from conventional neuroleptics to olanzapine. Biol Psychiatry. 2002;51:493–7.
Ota M, Wakabayashi C, Sato N, Hori H, Hattori K, Teraishi T, et al. Effect of l-theanine on glutamatergic function in patients with schizophrenia. Acta Neuropsychiatrica. 2015;27:291–6.
Ertugrul A, Volkan-Salanci B, Basar K, Karli Oguz K, Demir B, Ergun EL, et al. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: relationship with treatment response. Psychiatry Res Neuroimaging. 2009;174:121–9.
Jarskog LF, Dong Z, Kangarlu A, Colibazzi T, Girgis RR, Kegeles LS, et al. Effects of davunetide on N-acetylaspartate and choline in dorsolateral prefrontal cortex in patients with schizophrenia. Neuropsychopharmacology. 2013;38:1245–52.
Cadena EJ, White DM, Kraguljac NV, Reid MA, Maximo JO, Nelson EA, et al. A longitudinal multimodal neuroimaging study to examine relationships between resting state glutamate and task related BOLD response in schizophrenia. Front Psychiatry. 2018;9:632.
Egerton A, Bhachu A, Merritt K, McQueen G, Szulc A, McGuire P. Effects of antipsychotic administration on brain glutamate in schizophrenia: a systematic review of longitudinal 1H-MRS studies. Front Psychiatry. 2017;8:66.
Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry. 2016;73:665–74.
Scotti-Muzzi E, Umla-Runge K, Soeiro-de-Souza MG. Anterior cingulate cortex neurometabolites in bipolar disorder are influenced by mood state and medication: a meta-analysis of 1H-MRS studies. Eur Neuropsychopharmacol. 2021;47:62–73.
Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117:347–68.
Fornito A, Yücel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35:973–93.
Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19–31.
Ren W, Lui S, Deng W, Li F, Li M, Huang X, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry. 2013;170:1308–16.
Hartmann JA, Nelson B, Ratheesh A, Treen D, McGorry PD. At-risk studies and clinical antecedents of psychosis, bipolar disorder and depression: a scoping review in the context of clinical staging. Psychol Med. 2019;49:177–89.
Tonna M, Ossola P, Marchesi C, Bettini E, Lasalvia A, Bonetto C, et al. Dimensional structure of first episode psychosis. Early Inter Psychiatry. 2019;13:1431–8.
Reininghaus U, Böhnke JR, Chavez-Baldini U, Gibbons R, Ivleva E, Clementz BA, et al. Transdiagnostic dimensions of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). World Psychiatry. 2019;18:67–76.
Chan SY, Brady RO, Lewandowski KE, Higgins A, Öngür D, Hall M-H. Dynamic and progressive changes in thalamic functional connectivity over the first five years of psychosis. Mol Psychiatry. https://doi.org/10.1038/s41380-021-01319-3 2021.
Jauhar S, Veronese M, Nour MM, Rogdaki M, Hathway P, Turkheimer FE, et al. Determinants of treatment response in first-episode psychosis: an 18F-DOPA PET study. Mol Psychiatry. 2019;24:1502–12.
Kim S, Shin SH, Santangelo B, Veronese M, Kang SK, Lee JS, et al. Dopamine dysregulation in psychotic relapse after antipsychotic discontinuation: an [18F]DOPA and [11C]raclopride PET study in first-episode psychosis. Mol Psychiatry. https://doi.org/10.1038/s41380-020-00879-0 2020.
Kraguljac NV, Anthony T, Morgan CJ, Jindal RD, Burger MS, Lahti AC. White matter integrity, duration of untreated psychosis, and antipsychotic treatment response in medication-naïve first-episode psychosis patients. Mol Psychiatry. 2021;26:5347–56.
Lesh TA, Maddock RJ, Howell A, Wang H, Tanase C, Daniel Ragland J, et al. Extracellular free water and glutathione in first-episode psychosis-a multimodal investigation of an inflammatory model for psychosis. Mol Psychiatry. 2021;26:761–71.
Kübler U. Structured Clinical Interview for DSM-IV (SCID). In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine. New York, NY: Springer; 2013. p. 1919–20.
Jäger M, Bottlender R, Strauss A, Möller H-J. On the descriptive validity of ICD-10 schizophrenia: empirical analyses in the spectrum of non-affective functional psychoses. Psychopathology. 2003;36:152–9.
Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. 2010;12:638–46.
Amoretti S, Cabrera B, Torrent C, Mezquida G, Lobo A, González-Pinto A, et al. Cognitive reserve as an outcome predictor: first-episode affective versus non-affective psychosis. Acta Psychiatr Scand. 2018;138:441–55.
Chung J, Miller BJ. Meta-analysis of comorbid diabetes and family history of diabetes in non-affective psychosis. Schizophr Res. 2020;216:41–47.
Yung NCL, Wong CSM, Chan JKN, Chen EYH, Chang WC. Excess mortality and life-years lost in people with schizophrenia and other non-affective psychoses: an 11-year population-based cohort study. Schizophr Bull. 2021;47:474–84.
Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–11.
Versluis MJ, Kan HE, van Buchem MA, Webb AG. Improved signal to noise in proton spectroscopy of the human calf muscle at 7 T using localized B1 calibration. Magn Reson Med. 2010;63:207–11.
Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9.
Soher B, Semanchuk P, Todd D, Steinberg J, Young K. Vespa: Integrated applications for RF pulse design, spectral simulation and MRS data analysis. Proc. Intl. Soc. Mag. Reson. Med. 2011;19:1410.
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35.
Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, et al. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neurosci. 2014;34:8488–98.
Harrison TM, Joie RL, Maass A, Baker SL, Swinnerton K, Fenton L, et al. Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Ann Neurol. 2019;85:229–40.
Hayes JF, Marston L, Walters K, King MB, Osborn DPJ. Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000–2014. Br J Psychiatry. 2017;211:175–81.
Krogsrud SK, Fjell AM, Tamnes CK, Grydeland H, Mork L, Due-Tønnessen P, et al. Changes in white matter microstructure in the developing brain—A longitudinal diffusion tensor imaging study of children from 4 to 11years of age. NeuroImage. 2016;124:473–86.
Jarosz A, Wiley J. What are the odds? A practical guide to computing and reporting bayes factors. J Probl Solving. 2014;7:2.
Leucht S, Samara M, Heres S, Davis JM. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42:S90–94. Suppl 1.
Brady RO, Cooper A, Jensen JE, Tandon N, Cohen B, Renshaw P, et al. A longitudinal pilot proton MRS investigation of the manic and euthymic states of bipolar disorder. Transl Psychiatry. 2012;2:e160.
Nery FG, Weber WA, Blom TJ, Welge J, Patino LR, Strawn JR, et al. Longitudinal proton spectroscopy study of the prefrontal cortex in youth at risk for bipolar disorder before and after their first mood episode. Bipolar Disord. 2019;21:330–41.
Palaniyappan L. Progressive cortical reorganisation: a framework for investigating structural changes in schizophrenia. Neurosci Biobehav Rev. 2017;79:1–13.
Fusar-Poli P, Broome MR, Woolley JB, Johns LC, Tabraham P, Bramon E, et al. Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: longitudinal VBM-fMRI study. J Psychiatr Res. 2011;45:190–8.
Koo M-S, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry. 2008;65:746–60.
Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27.
Miyata J. Toward integrated understanding of salience in psychosis. Neurobiol Dis. 2019;131:104414.
Takahashi T, Kido M, Sasabayashi D, Nakamura M, Furuichi A, Takayanagi Y, et al. Gray matter changes in the insular cortex during the course of the schizophrenia spectrum. Front Psychiatry. 2020;11:659.
Li M, Li X, Das TK, Deng W, Li Y, Zhao L, et al. Prognostic utility of multivariate morphometry in schizophrenia. Front Psychiatry. 2019;10:245.
Liloia D, Brasso C, Cauda F, Mancuso L, Nani A, Manuello J, et al. Updating and characterizing neuroanatomical markers in high-risk subjects, recently diagnosed and chronic patients with schizophrenia: a revised coordinate-based meta-analysis. Neurosci Biobehav Rev. 2021;123:83–103.
Aoyama N, Théberge J, Drost DJ, Manchanda R, Northcott S, Neufeld RWJ, et al. Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry. 2011;198:448–56.
Li J, Ren H, He Y, Li Z, Ma X, Yuan L, et al. Anterior cingulate cortex glutamate levels are related to response to initial antipsychotic treatment in drug-naive first-episode schizophrenia patients. Front Psychiatry. 2020;11:553269.
Marsman A, Mandl RCW, van den Heuvel MP, Boer VO, Wijnen JP, Klomp DWJ, et al. Glutamate changes in healthy young adulthood. Eur Neuropsychopharmacol. 2013;23:1484–90.
Kaminski J, Mascarell-Maricic L, Fukuda Y, Katthagen T, Heinz A, Schlagenhauf F. Glutamate in the dorsolateral prefrontal cortex in patients with schizophrenia: a meta-analysis of 1H-magnetic resonance spectroscopy studies. Biol Psychiatry. 2021;89:270–7.
Cleeland C, Pipingas A, Scholey A, White D. Neurochemical changes in the aging brain: a systematic review. Neurosci Biobehav Rev. 2019;98:306–19.
Roalf DR, Sydnor VJ, Woods M, Wolk DA, Scott JC, Reddy R, et al. A quantitative meta-analysis of brain glutamate metabolites in aging. Neurobiol Aging. 2020;95:240–9.
Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–59.
Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry. 2016;21:198–204.
Miyake M, Kakimoto Y, Sorimachi M. A gas chromatographic method for the determination of N-acetyl-L-aspartic acid, N-acetyl-alpha-aspartylglutamic acid and beta-citryl-L-glutamic acid and their distributions in the brain and other organs of various species of animals. J Neurochem. 1981;36:804–10.
Rosenberg RN, Pascual JM. Rosenberg’s molecular and genetic basis of neurological and psychiatric disease. 5th ed. Elsevier, London; 2015.
Du F, Cooper AJ, Thida T, Shinn AK, Cohen BM, Öngür D. Myelin and axon abnormalities in schizophrenia measured with magnetic resonance imaging techniques. Biol Psychiatry. 2013;74:451–7.
Zong X, Hu M, Li Z, Cao H, He Y, Liao Y, et al. N-acetylaspartate reduction in the medial prefrontal cortex following 8 weeks of risperidone treatment in first-episode drug-naïve schizophrenia patients. Sci Rep. 2015;5:9109.
Gillaspy GE. The cellular language of myo-inositol signaling. N Phytol. 2011;192:823–39.
Harris JL, Choi I-Y, Brooks WM. Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front Aging Neurosci. 2015;7:202.
Das TK, Dey A, Sabesan P, Javadzadeh A, Théberge J, Radua J, et al. Putative astroglial dysfunction in schizophrenia: a meta-analysis of 1H-MRS studies of medial prefrontal myo-inositol. Front Psychiatry. 2018;9:438.
Freedman R, Ross RG. Prenatal choline and the development of schizophrenia. Shanghai Arch Psychiatry. 2015;27:90–102.
Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–43.
Ongür D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. 2009;172:44–48.
Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, et al. Neurometabolites in schizophrenia and bipolar disorder – A systematic review and meta-analysis. Psychiatry Res. 2012;203:111–25.
Ramírez-Expósito MJ, Martínez-Martos JM. The delicate equilibrium between oxidants and antioxidants in brain glioma. Curr Neuropharmacol. 2019;17:342–51.
Pan Y, Dempster K, Jeon P, Théberge J, Khan AR, Palaniyappan L. Acute conceptual disorganization in untreated first-episode psychosis: a combined magnetic resonance spectroscopy and diffusion imaging study of the cingulum. J Psychiatry Neurosci. 2021;46:E337–E346.
Das TK, Javadzadeh A, Dey A, Sabesan P, Théberge J, Radua J, et al. Antioxidant defense in schizophrenia and bipolar disorder: a meta-analysis of MRS studies of anterior cingulate glutathione. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:94–102.
Wood SJ, Berger GE, Wellard RM, Proffitt T-M, McConchie M, Berk M, et al. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiol Dis. 2009;33:354–7.
Sydnor VJ, Roalf DR. A meta-analysis of ultra-high field glutamate, glutamine, GABA and glutathione 1HMRS in psychosis: Implications for studies of psychosis risk. Schizophr Res. 2020;226:61–69.
Coughlin JM, Yang K, Marsman A, Pradhan S, Wang M, Ward RE, et al. A multimodal approach to studying the relationship between peripheral glutathione, brain glutamate, and cognition in health and in schizophrenia. Mol Psychiatry. 2021;26:3502–11.
Xin L, Mekle R, Fournier M, Baumann PS, Ferrari C, Alameda L, et al. Genetic polymorphism associated prefrontal glutathione and its coupling with brain glutamate and peripheral redox status in early psychosis. Schizophr Bull. 2016;42:1185–96.
Yang K, Longo L, Narita Z, Cascella N, Nucifora FC, Coughlin JM, et al. A multimodal study of a first episode psychosis cohort: potential markers of antipsychotic treatment resistance. Mol Psychiatry. 2022;27:1184–91.
Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol Psychiatry. 2016;21:10–28.
Cabungcal J-H, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–84.
Sawa A, Seidman LJ. Is prophylactic psychiatry around the corner? Combating adolescent oxidative stress for adult psychosis and schizophrenia. Neuron. 2014;83:991–3.
Acknowledgements
This study is supported by The National Institute of Mental Health Grants MH-092443 (to AS), MH-094268 (to AS), MH-105660 (to AS), and MH-107730 (to AS); foundation grants from Stanley (to AS), RUSK/S-R (to AS), and a NARSAD young investigator award from the Brain and Behavior Research Foundation (to AS, KY). Study recruitment was in part funded by Mitsubishi Tanabe Pharma Corporation, Japan. LP acknowledges support from the Tanna Schulich Chair of Neuroscience and Mental Health. The authors thank Yukiko Lema for suggestions, for formatting the figures and for her role in research management, and thank Dr. Melissa A Landek-Salgado for scientific and English editions.
Author information
Authors and Affiliations
Contributions
The current research was designed by AS, PBB, and KY. The analytic pipeline was designed by KY, LY, and DG. The data was analyzed by MW and KY. Analysis results interpretation were assisted by NGC and LP. Clinical recruitment is supervised by AS. Study participants were recruited and/or interviewed by NGC, JMC, GN, FCN, TWS, and AK. The manuscript was drafted by MW, KY, AS and PBB. All authors contributed to the discussion of the results and have approved the final manuscript to be published.
Corresponding authors
Ethics declarations
Competing interests
LP receives book royalties from Oxford University Press and income from the SPMM MRCPsych course. LP has received investigator-initiated educational grants from Otsuka, Janssen, and Sunovion Canada and speaker fees from Otsuka and Janssen Canada, and the Canadian Psychiatric Association. The original recruitment of study participants was partly funded by Mitsubishi Tanabe Pharma Corporation. However, this company is not involved in this specific study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Barker, P.B., Cascella, N.G. et al. Longitudinal changes in brain metabolites in healthy controls and patients with first episode psychosis: a 7-Tesla MRS study. Mol Psychiatry 28, 2018–2029 (2023). https://doi.org/10.1038/s41380-023-01969-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-01969-5
This article is cited by
-
Reduction of N-acetyl aspartate (NAA) in association with relapse in early-stage psychosis: a 7-Tesla MRS study
Schizophrenia (2024)
-
Psychosis and autism spectrum disorder: a special issue of Molecular Psychiatry
Molecular Psychiatry (2023)
-
Magnetic Resonance Spectroscopy Studies of Brain Energy Metabolism in Schizophrenia: Progression from Prodrome to Chronic Psychosis
Current Psychiatry Reports (2023)