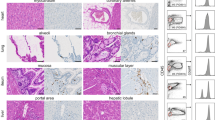
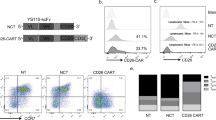
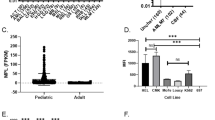
Abstract
The only current curative treatment for chronic lymphocytic leukemia (CLL) is allogenic hematopoietic stem cell transplantation. Chimeric antigen receptor treatment targeting CD19 for CLL achieved some complete responses, suggesting the need for alternative or combinational therapies to achieve a more robust response. In this work, we evaluated CAR-T cells specific for Siglec-6, an antigen expressed in CLL, as a novel CAR-T cell treatment for CLL. We found that detection of SIGLEC6 mRNA and Siglec-6 protein is highly restricted to placenta and immune cells in other tissues and it is not expressed in hematopoietic stem cells. We generated CAR-T cells specific for Siglec-6 based on the sequence of the fully human anti-Siglec-6 antibody (JML1), which was identified in a CLL patient that was cured after allo-hematopoietic stem cell transplantation (alloHSCT), and observed that it specifically targeted CLL cells in vitro and in a xenograft mouse model. Interestingly, a short hinge region increased the activity of CAR-T cells to target cells expressing higher Siglec-6 levels but similarly targeted CLL cells expressing lower Siglec-6 levels in vitro and in vivo. Our results identify a novel CAR-T cell therapy for CLL and establish Siglec-6 as a possible target for immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–15.
Kipps TJ, Stevenson FK, Wu CJ, Croce CM, Packham G, Wierda WG, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Prim. 2017;3:17008.
Wiestner A. The role of B-cell receptor inhibitors in the treatment of patients with chronic lymphocytic leukemia. Haematologica. 2015;100:1495–507.
Ahn IE, Underbayev C, Albitar A, Herman SE, Tian X, Maric I, et al. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood. 2017;129:1469–79.
Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–94.
Boyiadzis M, Foon KA, Pavletic S. Hematopoietic stem cell transplantation for chronic lymphocytic leukemia: potential cure for an incurable disease. Exp Opin Biol Ther. 2007;7:1789–97.
Mato A, Porter DL. A drive through cellular therapy for CLL in 2015: allogeneic cell transplantation and CARs. Blood. 2015;126:478–85.
Majzner RG, Mackall CL. Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 2019;25:1341–55.
Ruella M, Maus MV. Catch me if you can: leukemia escape after CD19-Directed T cell immunotherapies. Comput Struct Biotechnol J. 2016;14:357–62.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17.
Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–26.
O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharm Sci. 2009;30:240–8.
Pehlivan KC, Duncan BB, Lee DW. CAR-T cell therapy for acute lymphoblastic leukemia: transforming the treatment of relapsed and refractory disease. Curr Hematol Malig Rep. 2018;13:396–406.
Rumer KK, Uyenishi J, Hoffman MC, Fisher BM, Winn VD. Siglec-6 expression is increased in placentas from pregnancies complicated by preterm preeclampsia. Reprod Sci. 2013;20:646–53.
Yu Y, Blokhuis BRJ, Diks MAP, Keshavarzian A, Garssen J, Redegeld FA. Functional inhibitory Siglec-6 is upregulated in human colorectal cancer-associated mast cells. Front Immunol. 2018;9:2138.
Kardava L, Moir S, Wang W, Ho J, Buckner CM, Posada JG, et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Investig. 2011;121:2614–24.
Chang J, Peng H, Shaffer BC, Baskar S, Wecken IC, Cyr MG, et al. Siglec-6 on chronic lymphocytic leukemia cells is a target for post-allogeneic hematopoietic stem cell transplantation antibodies. Cancer Immunol Res. 2018;6:1008–13.
Baskar S, Suschak JM, Samija I, Srinivasan R, Childs RW, Pavletic SZ, et al. A human monoclonal antibody drug and target discovery platform for B-cell chronic lymphocytic leukemia based on allogeneic hematopoietic stem cell transplantation and phage display. Blood. 2009;114:4494–502.
Kochenderfer JN, Feldman SA, Zhao Y, Xu H, Black MA, Morgan RA, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32:689–702.
Hermans IF, Silk JD, Yang J, Palmowski MJ, Gileadi U, McCarthy C, et al. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J Immunol Methods. 2004;285:25–40.
Patel N, Brinkman-Van der Linden EC, Altmann SW, Gish K, Balasubramanian S, Timans JC, et al. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J Biol Chem. 1999;274:22729–38.
Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O’Neill A, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005;28:203–11.
Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19:3153–64.
Peng H, Nerreter T, Chang J, Qi J, Li X, Karunadharma P, et al. Mining naive rabbit antibody repertoires by phage display for monoclonal antibodies of therapeutic utility. J Mol Biol. 2017;429:2954–73.
Srivastava S, Riddell SR. Engineering CAR-T cells: design concepts. Trends Immunol. 2015;36:494–502.
Acknowledgements
We thank Dr. James N. Kochenderfer for providing the CD19-CAR construct. This work was supported by an NIH, Bench to Bedside and back Program (Grant ID: 600897) to DK, REG, SP, and CR. CR also acknowledges funding by NIH grant R21 CA229961.
Author information
Authors and Affiliations
Contributions
DK supervised, performed experiments, and wrote the manuscript, JHY, SS, EV, MGC performed experiments, AW provided the primary CLL samples and scientific expertise, CR developed monoclonal antibody JML1, supervised experiments, edited the manuscript, and provided scientific expertise, JA and SP performed immunohistochemistry, and REG provided scientific guidance.
Corresponding author
Ethics declarations
Conflict of interest
CR is an inventor of U.S. Patent 8,877,199 which claims monoclonal antibody JML1. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kovalovsky, D., Yoon, J.H., Cyr, M.G. et al. Siglec-6 is a target for chimeric antigen receptor T-cell treatment of chronic lymphocytic leukemia. Leukemia 35, 2581–2591 (2021). https://doi.org/10.1038/s41375-021-01188-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01188-3
This article is cited by
-
The intriguing roles of Siglec family members in the tumor microenvironment
Biomarker Research (2022)
-
Tumor buster - where will the CAR-T cell therapy ‘missile’ go?
Molecular Cancer (2022)