Abstract
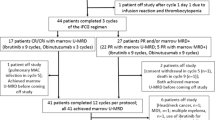
An immunosuppressive microenvironment promoting leukemia cell immune escape plays an important role in the pathogenesis of AML. Through its interaction with cereblon, a substrate receptor for the E3 ubiquitin ligase complex, pomalidomide leads to selective ubiquitination of transcription factors Aiolos and Ikaros thereby promoting immune modulation. In this phase I trial, 51 newly diagnosed non-favorable risk AML and high-risk MDS patients were enrolled and treated with AcDVP16 (cytarabine 667 mg/m2/day IV continuous infusion days 1–3, daunorubicin 45 mg/m2 IV days 1–3, etoposide 400 mg/m2 IV days 8–10) induction therapy followed by dose- and duration-escalation pomalidomide beginning at early lymphocyte recovery. Forty-three patients (AML: n = 39, MDS: n = 4) received pomalidomide. The maximum tolerated dose of pomalidomide was 4 mg for 21 consecutive days. The overall complete remission (CR + CRi) rate, median overall survival, and disease-free survival were 75%, 27.1 and 20.6 months, respectively. Subset analyses revealed 86% CR/CRi rate in AML patients with unfavorable-risk karyotype treated with pomalidomide. Pomalidomide significantly decreased Aiolos expression in both CD4+ and CD8+ peripheral blood and bone marrow T cells, promoted T cell differentiation, proliferation, and heightened their cytokine production. Finally, pomalidomide induced distinct gene expression changes in immune function-related ontologies in CD4+ and CD8+ T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Burnett AK. Treatment of acute myeloid leukemia: are we making progress? Hematol Am Soc Hematol Educ Program. 2012;2012:1–6.
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5.
Karp JE, Garrett-Mayer E, Estey EH, Rudek MA, Smith BD, Greer JM, et al. Randomized phase II study of two schedules of flavopiridol given as timed sequential therapy with cytosine arabinoside and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Haematologica. 2012;97:1736–42.
Zeidner JF, Foster MC, Blackford AL, Litzow MR, Morris LE, Strickland SA, et al. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100:1172–9.
Castaigne S, Chevret S, Archimbaud E, Fenaux P, Bordessoule D, Tilly H, et al. Randomized comparison of double induction and timed-sequential induction to a “3 + 7” induction in adults with AML: long-term analysis of the Acute Leukemia French Association (ALFA) 9000 study. Blood. 2004;104:2467–74.
Norsworthy KJ, DeZern AE, Tsai HL, Hand WA, Varadhan R, Gore SD, et al. Timed sequential therapy for acute myelogenous leukemia: Results of a retrospective study of 301 patients and review of the literature. Leuk Res. 2017;61:25–32.
Knaus HA, Kanakry CG, Luznik L, Gojo I. Immunomodulatory drugs II: immune checkpoint agents in acute leukemia. Curr Drug Targets. 2017;18:315–31.
Davidson-Moncada J, Viboch E, Church SE, Warren SE, Rutella S. Dissecting the Immune Landscape of Acute Myeloid Leukemia. Biomedicines. 2018;6.
Knaus HA, Berglund S, Hackl H, Blackford AL, Zeidner JF, Montiel-Esparza R, et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight. 2018;3:e120974.
Kong Y, Zhang J, Claxton DF, Ehmann WC, Rybka WB, Zhu L, et al. PD-1(hi)TIM-3(+) T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation. Blood Cancer J. 2015;5:e330.
Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC, et al. T cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res. 2016;22:3057–66.
Wang X, Zheng J, Liu J, Yao J, He Y, Li X, et al. Increased population of CD4(+)CD25(high), regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol. 2005;75:468–76.
Han Y, Dong Y, Yang Q, Xu W, Jiang S, Yu Z, et al. Acute myeloid leukemia cells express ICOS ligand to promote the expansion of regulatory T cells. Front Immunol. 2018;9:2227.
Ersvaer E, Liseth K, Skavland J, Gjertsen BT, Bruserud O. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC Immunol. 2010;11:38.
Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A, et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res. 2009;15:3325–32.
Kanakry CG, Hess AD, Gocke CD, Thoburn C, Kos F, Meyer C, et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood. 2011;117:608–17.
Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–45.
Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187:1885–92.
LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–90.
Shanafelt TD, Ramsay AG, Zent CS, Leis JF, Tun HW, Call TG, et al. Long-term repair of T cell synapse activity in a phase II trial of chemoimmunotherapy followed by lenalidomide consolidation in previously untreated chronic lymphocytic leukemia (CLL). Blood. 2013;121:4137–41.
Ramsay AG, Evans R, Kiaii S, Svensson L, Hogg N, Gribben JG. Chronic lymphocytic leukemia cells induce defective LFA-1-directed T cell motility by altering Rho GTPase signaling that is reversible with lenalidomide. Blood. 2013;121:2704–14.
Zeidner JF, Foster MC. Immunomodulatory drugs: IMiDs in acute myeloid leukemia (AML). Curr Drug Targets. 2017;18:304–14.
Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G, et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009-02. Blood. 2013;121:1968–75.
Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164:811–21.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–35.
Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84.
Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Investig. 2011;121:2350–60.
Bachireddy P, Hainz U, Rooney M, Pozdnyakova O, Aldridge J, Zhang W, et al. Reversal of in situ T cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood. 2014;123:1412–21.
Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med. 2018;24:978–85.
Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clinical Oncol. 2018:Jco2017776112.
Richardson PG, Siegel D, Baz R, Kelley SL, Munshi NC, Laubach J, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121:1961–7.
Le Roy A, Prebet T, Castellano R, Goubard A, Riccardi F, Fauriat C, et al. Immunomodulatory drugs exert anti-leukemia effects in acute myeloid leukemia by direct and immunostimulatory activities. Front Immunol. 2018;9:977.
Stone RM, Mazzola E, Neuberg D, Allen SL, Pigneux A, Stuart RK, et al. Phase III open-label randomized study of cytarabine in combination with amonafide L-malate or daunorubicin as induction therapy for patients with secondary acute myeloid leukemia. J Clin Oncol. 2015;33:1252–7.
Luskin MR, Lee JW, Fernandez HF, Abdel-Wahab O, Bennett JM, Ketterling RP, et al. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood. 2016;127:1551–8.
Zeidan AM, Smith BD, Carraway HE, Gojo I, DeZern A, Gore SD. A phase 2 trial of high dose lenalidomide in patients with relapsed/refractory higher-risk myelodysplastic syndromes and acute myeloid leukaemia with trilineage dysplasia. Br J Haematol. 2017;176:241–7.
Medeiros BC, McCaul K, Kambhampati S, Pollyea DA, Kumar R, Silverman LR, et al. Randomized study of continuous high-dose lenalidomide, sequential azacitidine and lenalidomide, or azacitidine in persons 65 years and over with newly-diagnosed acute myeloid leukemia. Haematologica. 2018;103:101–6.
Ades L, Prebet T, Stamatoullas A, Recher C, Guieze R, Raffoux E, et al. Lenalidomide combined with intensive chemotherapy in acute myeloid leukemia and higher-risk myelodysplastic syndrome with 5q deletion. Results of a phase II study by the Groupe Francophone Des Myelodysplasies. Haematologica. 2017;102:728–35.
Dennis M, Culligan D, Karamitros D, Vyas P, Johnson P, Russell NH, et al. Lenalidomide monotherapy and in combination with cytarabine, daunorubicin and etoposide for high-risk myelodysplasia and acute myeloid leukaemia with chromosome 5 abnormalities. Leuk Res Rep. 2013;2:70–4.
Bansal D, Vij K, Chang GS, Miller CA, DiPersio JF, Vij R, et al. Lenalidomide results in a durable complete remission in acute myeloid leukemia accompanied by persistence of somatic mutations and a T cell infiltrate in the bone marrow. Haematologica. 2018;103:e270–e3.
Clave E, Douay C, Coman T, Busson M, Bompoint C, Moins-Teisserenc H, et al. Lenalidomide consolidation and maintenance therapy after autologous stem cell transplant for multiple myeloma induces persistent changes in T cell homeostasis. Leuk lymphoma. 2014;55:1788–95.
McDaniel JM, Zou JX, Fulp W, Chen DT, List AF, Epling-Burnette PK. Reversal of T cell tolerance in myelodysplastic syndrome through lenalidomide immune modulation. Leukemia. 2012;26:1425–9.
Acknowledgements
This data was presented at the annual American Society of Hematology conference in 2015 in Orlando, FL (poster), 2016 in San Diego, CA (poster), and 2018 in San Diego, CA (oral presentation). This study was supported in part by research funding from UM1-CA186691 and P30 CA006973 (Johns Hopkins), 5-UM1-CA186704 (University of North Carolina), and P30CA016359/5-UM1-CA186689-5 (Yale). JFZ was supported and funded by Leukemia and Lymphoma Society Special Fellow in Clinical Research Award. HAK was supported by ASBMT New Investigator Award/Gabrielle’s Angel Foundation and by the Vienna Fund for Innovative Cancer Research. AMZ is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA). The authors would like to thank the research support staff at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, University of North Carolina, Lineberger Comprehensive Cancer Center, and Yale Cancer Center. We would also like to thank all of the patients and their families who trusted us for their care. Without these partnerships, this trial could not have been performed.
Author information
Authors and Affiliations
Contributions
JFZ, HAK, LL, and IG designed research, performed research, collected data, analyzed and interpreted data, and wrote the manuscript. AMZ, RME, GTP, LPG, GG, MMS, AED, KWP, BDS, MJL, SG, CCC, and MCF performed research. ALB and HH analyzed and interpreted the data, and performed statistical analysis. HS designed research. JEK designed research and wrote the manuscript.
Funding
This study was supported in part by research funding from UM1-CA186691 and P30 CA006973 (Johns Hopkins), 5-UM1-CA186704 (University of North Carolina), and P30CA016359/5-UM1-CA186689-5 (Yale). JFZ was supported and funded by Leukemia and Lymphoma Society Special Fellow in Clinical Research Award. HAK was supported by ASBMT New Investigator Award/Gabrielle’s Angel Foundation and by the Vienna Fund for Innovative Cancer Research. AMZ is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA).
Corresponding author
Ethics declarations
Conflict of interest
JFZ reports receiving research funding from Celgene, Merck, Takeda, and Tolero Pharmaceuticals, honoraria from AbbVie, Agios, Celgene, Daiichi Sankyo, Genentech, Pfizer, and Tolero Pharmaceuticals, and has served as a consultant for AsystBio Laboratories, Celgene, Covance, and Takeda. AMZ reports receiving research funding (institutional) from Celgene, Acceleron, Abbvie, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, and ADC Therapeutics, consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Jazz, Celgene, Ariad, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, BeyondSpring, and Takeda. None of these relationships were related to the development of this manuscript. BDS reports being on Data Safety Monitoring Board for Celgene and has served as a consultant for Agios, Bristol-Myers Squibb, Jazz, Novartis, and Pfizer. MJL reports receiving research funding from Astellas, FujiFilm, and Novartis, and has served as a consultant for Agios, Amgen, Astellas, Daiichi Sankyo, FujiFilm, Karyopharm, Menarini, and Novartis. CCC has received honoraria from Abbvie, Loxo, Pharmacyclics, Octapharma, and H3 Biomedicine, has served as a consultant for Abbvie, Covance, and Cowen & Co., and has received institutional funding from Incyte, Gilead, AROG, Loxo, and H3 Biomedicine. LL has received research support from Genentech and Merck and has a patent licensed to WindMIL Therapeutics (WO2014085437A2). IG received research funding from Merck and Amgen, and participated in the advisory board meetings for Amgen, Jazz, Novartis and Abbvie. None of these relationships were related to the development of this manuscript. All other authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zeidner, J.F., Knaus, H.A., Zeidan, A.M. et al. Immunomodulation with pomalidomide at early lymphocyte recovery after induction chemotherapy in newly diagnosed AML and high-risk MDS. Leukemia 34, 1563–1576 (2020). https://doi.org/10.1038/s41375-019-0693-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0693-4
This article is cited by
-
Single-cell transcriptomic profiling reveals immune cell heterogeneity in acute myeloid leukaemia peripheral blood mononuclear cells after chemotherapy
Cellular Oncology (2024)
-
Progress on biphenyl derivatives as PD-1/PD-L1 inhibitors
Medicinal Chemistry Research (2023)