Abstract
Background
Caffeine citrate is the most frequently used medication in preterm neonates for the prevention of apnea of prematurity. There is no accepted consensus regarding the optimal caffeine citrate dosing. In this study, we evaluate clinical responses of premature neonates to standard-dose caffeine citrate treatment.
Methods
A prospective observational study conducted at the NICU at Sheba Medical Center (3/2016-2/2017). The study population included preterm neonates born at a gestational age (GA) < 33 weeks and treated with caffeine citrate according to the local NICU protocol.
Results
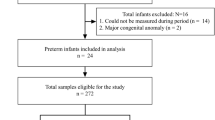
The study cohort included 66 preterm neonates of GA < 33 weeks. Thirty infants were defined as responders and 36 as nonresponders to 7.5 mg/kg caffeine citrate treatment, and they required a further dose increase to 10 mg/kg. Infants in the nonresponders group were born at earlier GA than responders (29 vs. 31 weeks, respectively, P = 0.004). The nonresponders required a significantly longer hospital stay (56 vs. 46 days, P = 0.014), and longer supplemental oxygen support (18 vs 2 days, P = 0.008).
Conclusions
Caffeine citrate initiation at higher doses is safe and does not require routine serum levels monitoring. It might be more effective for controlling apnea of prematurity in preterm neonates born ≤29 weeks of gestation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All the data presented in the manuscript is available upon request from the corresponding author.
References
Natarajan G, Lulic-Botica M, Aranda J. Pharmacology review: clinical pharmacology of caffeine in the newborn. NeoReviews. 2007;8:e214–21.
Dobson NR, Hunt CE. Pharmacology review: caffeine use in neonates: indications, pharmacokinetics, clinical effects, outcomes. NeoReviews. 2013;14:e540–50.
Henderson‐Smart DJ, De Paoli AG. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst. Rev. 2010.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med 2006;354:2112–21.
Kou C, Han D, Li Z, Wu W, Liu Z, Zhang Y, et al. Influence of prevention of caffeine citrate on cytokine profile and bronchopulmonary dysplasia in preterm infants with apnea. Minerva Pediatr. 2020;72:95–100.
Henderson‐Smart DJ, Davis PG. Prophylactic methylxanthines for endotracheal extubation in preterm infants. Cochrane Database Syst Rev. 2010.
Rossor T, Bhat R, Ali K, Peacock J, Rafferty GF, Greenough A. The effect of caffeine on the ventilatory response to hypercarbia in preterm infants. Pediatr Res. 2018;83:1152.
Huvanandana J, Thamrin C, McEwan AL, Hinder M, Tracy MB. Cardiovascular impact of intravenous caffeine in preterm infants. Acta Paediatrica. 2019;108:423–9.
Aviles-Otero N, Kumar R, Khalsa DD, Green G, Carmody JB. Caffeine exposure and acute kidney injury in premature infants with necrotizing enterocolitis and spontaneous intestinal perforation. Pediatr Nephrol. 2019;34:729–36.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–902.
Lodha A, Entz R, Synnes A, Creighton D, Yusuf K, Lapointe A, et al. Early Caffeine Administration and Neurodevelopmental Outcomes in Preterm Infants. Pediatrics. 2019;143.
Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307:275–82.
Shepherd E, Salam RA, Middleton P, Han S, Makrides M, McIntyre S, et al. Neonatal interventions for preventing cerebral palsy: an overview of Cochrane Systematic Reviews. Cochrane Database Syst Rev. 2018;6.
Shrestha B, Jawa G. Caffeine citrate–Is it a silver bullet in neonatology? Pediatrics Neonatol. 2017;58:391–7.
Natarajan G, Botica ML, Thomas R, Aranda JV. Therapeutic drug monitoring for caffeine in preterm neonates: an unnecessary exercise? Pediatrics. 2007;119:936–40.
Charles BG, Townsend SR, Steer PA, Flenady VJ, Gray PH, Shearman A. Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Ther Drug Monit. 2008;30:709–16.
Chen J, Jin L, Chen X. Efficacy and safety of different maintenance doses of caffeine citrate for treatment of apnea in premature infants: a systematic review and meta-analysis. BioMed Res Int. 2018;2018:9061234. https://doi.org/10.1155/2018/9061234.
American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914–7.
Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr respiratory Rev. 2004;5:S377–82.
Kumral A, Tuzun F, Yesilirmak DC, Duman N, Ozkan H. Genetic basis of apnoea of prematurity and caffeine treatment response: role of adenosine receptor polymorphisms: genetic basis of apnoea of prematurity. Acta Paediatrica. 2012;101:e299–303.
Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern-fetal neonatal Med. 2010;23:199–203.
Ji S, Zhou W, Li X, Liu S, Wang F, Li X, et al. Maternal hyperglycemia disturbs neocortical neurogenesis via epigenetic regulation in C57BL/6J mice. Cell Death Dis. 2019;10:211.
Bauer J, Maier K, Linderkamp O, Hentschel R. Effect of caffeine on oxygen consumption and metabolic rate in very low birth weight infants with idiopathic apnea. Pediatrics 2001;107:660–3.
Author information
Authors and Affiliations
Contributions
CR designed the study, performed data collection; analyzed and interpreted the data and co-wrote the manuscript. CT assisted in data collection from patients’ files and managing nurses, assisted in drafting the initial manuscript; and analyzed the data. MH assisted in data collection and supervised the adherence to the caffeine citrate protocol and analyzed the data and assisted in drafting the initial manuscript. IG and RL assisted in study design, data collection, data analysis, and statistics. HY-B assisted in study design, data collection, data analysis, and statistics and co-wrote the manuscript. TS designed, coordinated, and conducted the study, including analysis and interpretation of data, and co-wrote the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study protocol was approved by the institutional review board of the Sheba Medical Center.
Consent to participate
The study cohort included 70 preterm neonates with a GA < 33 weeks whose parents signed informed written consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rosen, C., Taran, C., Hanna, M. et al. Caffeine citrate for apnea of prematurity—One dose does not fit all a prospective study. J Perinatol 41, 2292–2297 (2021). https://doi.org/10.1038/s41372-021-01172-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01172-w