Abstract
Objective
ABO hemolytic disease occurs among neonates with blood groups A or B delivered to group O women. Extreme neonatal hyperbilirubinemia due to ABO disease has been reported, but its frequency is not well known. We sought to determine the odds of developing severe ABO hemolytic disease in the 13 years since adopting universal bilirubin screening/management in the Intermountain Healthcare system.
Study design
We conducted a retrospective analysis of neonates born between 2004 and 2016, defining “severe hemolytic disease” as; (1) total serum bilirubin (TSB) >25 mg/dL, or (2) hospital readmission for jaundice, or (3) bilirubin encephalopathy. Neonates born to group O (+) mothers were included and considered either; (1) Controls (not at risk for ABO disease because they were group O), (2) Study subjects (at risk for ABO disease because they were group A or B).
Results
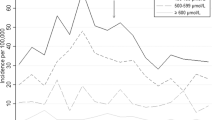
Of 400,531 live births, 47% were to group O women; 86% of whom were group O (+). Overall, 42,529 (27%) neonates born to group O (+) women had their blood group determined; 29,729 (68%) were O, 10,682 (25%) A, and 3109 (7%) B. Peak TSBs during the first 10 days were higher in group A (11.0 ± 4.2 mg/dL) and B (11.5 ± 4.3) than group O neonates (10.3 ± 4.1). However the relative risks of a TSB ≥25 mg/dL, readmission for jaundice, or kernicterus, were the same in the control vs. study groups.
Conclusions
In our health system, severe hemolytic disease in neonates born to group O (+) woman is not more likely in group A or B neonates than in controls (group O). We recognize that in other practices, particularly those who do not have a universal bilirubin screening/management program, ABO hemolytic disease severity might be different than in our system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ross ME, Waldron PE, Cashore WL, de Alarcon PA. Hemolytic disease of the fetus and newborn. In: de Alarcon PA, Werner EJ, Christensen RD, editors. Neonatal hematology. 2nd ed. Cambridge, UK: Cambridge University Press; 2013. p. 65–90.
Dufour DR, Monoghan WP. ABO hemolytic disease of the newborn: a retrospective analysis of 254 cases. Am J Clin Pathol. 1980;73:369–73.
Simmons DP, Savage WJ. Hemolysis from ABO incompatibility. Hematol Oncol Clin North Am. 2015;29:429–43.
Kaplan M, Hammerman C, Vreman HJ, Wong RJ, Stevenson DK. Hemolysis and hyperbilirubinemia in direct ABO blood group heterospecific neonates. J Pediatr. 2010;157:772–7.
Maisels MJ, Watchko JF. Routine blood typing and DAT in infants of group O mothers. J Perinatol. 2013;33:579.
Gilja BK, Shah VP. Hydrops fetalis due to ABO incompatibility. Clin Pediatr. 1988;27:210–2.
Drabik-Clary K, Reddy VVB, Benjamin WH, Boctor FN. Severe hemolytic disease of the newborn in a group B African-American infant delivered by a group O mother. Ann Clin & Lab Sci. 2006;36:205–7.
Miller DF, Petrie SJ. Fatal erythroblastosis fetalis secondary to ABO incompatibility. Report of a case. Obstet Gynecol. 1963;22:773–7.
Sherer DM, Abramozicz JS, Ryan RM, Sheils LA, Blumberg N, Woods JR Jr.. Severe fetal hydrops resulting from ABO incompatibility. Obstet Gynecol. 1991;78:897–9.
Stiller RJ, Herzlinger R, Siegel S, Whetham JVG. Fetal ascites associated with ABO incompatibility: Case report and review of the literature. Am J Obstet Gyenecol. 1996;175:1371–2.
McDonnell M, Hannam S, Devane SP. Hydrops fetalis due to ABO incompatibility. Arch Dis Child Fetal Neonatal Ed. 1998;78:F220–221.
Tiker F, Gurakan B, Tarcan A, Ozbek N. Fatal course of ABO hemolytic disease associated with hydrops in a twin pregnancy. Turk J Pediatr. 2006;48:73–75.
Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). J Perinatol. 2009;29 Suppl 1:S25–45.
Sgro M, Campbell DM, Kandasamy S, Shah V. Incidence of chronic bilirubin encephalopathy in Canada, 2007-8. Pediatrics. 2012;130:e886–e890.
Matthews DC, Glader B. Erythrocyte disorders in infancy. In: Gleason CA, Devaskar SU, editors. In Avery’s diseases of the newborn. 9th ed. Philadelphia, PA: Elsevier; 2012. p. 1091.
Diab Y, Luchtman-Jones L. Hematologic and oncologic problems in the fetus and neonate. In: Martin JG, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin’s Neonatal-Perinatal Medicine. 10th ed. Philadelphia, PA: Elsevier; 2015.
Koenig JM. Erythroblastosis fetalis. In: Christensen RD, editor. In hematologic problems of the neonate. Philadelphia: Saunders; 2000. p. 200–202.
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316.
Eggert LD, Wiedmeier SE, Wilson J, Christensen RD. The effect of instituting a prehospital-discharge newborn bilirubin screening program in an 18-hospital health system. Pediatrics. 2006;117:e855–862.
Bhutani VK, Johnson-Hammerman L. The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Fetal Neonatal Med. 2015;20:6–13.
Abrams ME, Meredith KS, Kinnard PA, Clark RH. Hydrops fetalis: retrospective review of cases reported to a large national database and identification of risk factors associated with death. Pediatrics. 2007;120:84–89.
Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from fa multihospital healthcare system. Pediatrics. 2009;123:e333–337.
Christensen RD, Henry E, Bennett ST, Yaish HM. Reference intervals for reticulocyte parameters of infants during their first 90 days after birth. J Perinatol. 2016;36:61–66.
Christensen RD, Henry E, Andres RL, Bennett ST. Reference ranges for blood concentrations of nucleated red blood cells in neonates. Neonatology. 2011;99:289–94.
Christensen RD, Yaish HM, Nussenzveig RH, Reading NS, Agarwal AM, Eggert LD, et al. Acute kernicterus in a neonate with O/B blood group incompatibility and a mutation in SLC4A1. Pediatrics. 2013;132:e531–534.
Christensen RD, Yaish HM, Wiedmeier SE, Reading NS, Pysher TJ, Palmer CA, et al. Neonatal death suspected to be from sepsis was found to be kernicterus with G6PD deficiency. Pediatrics. 2013;132:e1694–1698.
Singla S, Kumar S, Kumar RK, Sharma JB, Kachhawa G. Severe hydrops in the infant of a Rhesus D positive mother due to anti-c antibodies diagnosed antenatally: a case report. J Case Rep. 2010;4:57.
Hackney DN, Knudtson EJ, Rossi KQ, Krugh D, O’Shaughnessy RD. Management of pregnancies complicated by anti-c. Obstet Gynecol. 2004;103:24–30.
Sheeladevi CS, Suchitha S, Manjunath GV, Murthy S. Hemolytic disease of the newborn due to anti-c isoimmunization: A case report. Indian J Hematol Blood Transfus. 2013;29:155–7.
Christensen RD, Yaish HM, Johnson CB, Bianchi P, Zanella A. Six children with pyruvate kinase deficiency from one small town: molecular characterization of the PK-LR gene. J Pediatr. 2011;159:695–7.
Christensen RD, Nussenzveig RH, Yaish HM, Henry E, Eggert LD, Agarwal AM. Causes of hemolysis in neonates with extreme hyperbilirubinemia. J Perinatol. 2014;34:616–9.
Agarwal AM, Nussenzveig RH, Reading NS, Patel JL, Sangle N, Salama ME, et al. Clinical utility of next-generation sequencing in the diagnosis of hereditary haemolytic anaemias. Br J Haematol. 2016;174:806–11.
Sebert KE, Smith JW, Arnold DM, Chan HHW, Heddle NM, Kelton JG. Red Cell, platelet, and white cell antigens. In: Greer JP, Arber DA, Lander B, List AF, Means Jr RT, Paraskevas F, Rodgers GM, editors. Wintrobe’s Clinical Hematology. 13th ed. Philadelphia: Wolters Kluwer/Lippincott, Williams & Wilkins; 2014. p. 51.
Kalakheti BK, Singh R, Bhatta NK, Karki A, Baral N. Risk of neonatal hyperbilirubinemia in babies born to ‘O’ positive mothers: a prospective cohort study. Kathmandu Univ Med J. 2009;7:11–15.
Bhutani VK, Maisels MJ, Stark AR, Buonocore G, Expert Committee for Severe Neonatal Hyperbilirubinemia; European Society for Pediatric Research, American Academy of Pediatrics. Management of jaundice and prevention of severe neonatal hyperbilirubinemia in infants > or = 35 weeks gestation. Neonatology. 2008;94:63–67.
Judd WJ, for the Scientific Section Coordinating Committee of the AABB. Practice guidelines for prenatal and perinatal immunohematology, revisited. Transfusion. 2001;41:1445–52.
Kaplan M, Hammerman C, Vreman HJ, Womg RJ, Stevenson DK. Direct antiglobulin titer strength and hyperbilirubinemia. Pediatrics. 2014;134:e1340–1344.
Kristinsdottir T, Kjartansson S, Hardardottir H, Jonsson T, Halldorsdottir AM. Positive Coomb’s test in newborns; causes and clinical consequences Summary of cases diagnosed in the Blood Bank in the years 2005 to 2012. Laeknabladid. 2016;102:326–33.
Valsami S, Politou M, Boutsikou Τ, Briana D, Papatesta M, Malamitsi-Puchner A. Importance of direct antiglobulin test (DAT) in cord blood: causes of DAT (+) in a cohort study. Pediatr Neonatol. 2015;56:256–60.
Bhutani VK, Wong RJ, Vreman HJ, Stevenson DK, Jaundice Multinational Study Group. Bilirubin production and hour-specific bilirubin levels. J Perinatol. 2015;35:735–8.
Christensen RD, Lambert DK, Henry E, Yaish HM, Prchal JT. End-tidal carbon monoxide as an indicator of the hemolytic rate. Blood Cells Mol Dis. 2015;54:292–6.
Lozar-Krivec J, Bratanic B, Paro-Panjan D. The role of carboxyhemoglobin measured with CO-oximetry in the detection of hemolysis in newborns with ABO alloimmunization. J Matern Fetal Neonatal Med. 2016;29:452–6.
Christensen RD, Malleske DT, Lambert DK, Baer VL, Prchal JT, Denson DL, et al. Measuring end-tidal carbon monoxide of jaundiced neonates in the birth hospital to identify those with hemolysis. Neonatology. 2016;109:1–5.
Bhutani VK, Srinivas S, Castillo Cuadrado ME, Aby JL, Wong RJ, Stevenson DK. Identification of neonatal haemolysis: an approach to pre-discharge management of neonatal hyperbilirubinemia. Acta Paediatr. 2016;105:e189–194.
Tidmarsh GF, Wong RJ, Stevenson DK. End-tidal carbon monoxide and hemolysis. J Perinatol. 2014;34:577–81.
Watckho JF. Hyperbilirubinemia in African American neonates: clinical issues and current challenges. Semin Fetal Neonatal Med. 2010;15:176–82.
Akanmu AS, Oyedeji OA, Adeyemo TA, Ogbenna AA. Estimating the risk of ABO hemolytic disease of the newborn in Lagos. J Blood Transfus. 2015;2015:560738.
Oyedeji OA, Adeyemo TA, Ogbenna AA, Akanmu AS. Prevalence of anti-A and anti-B hemolysis among blood group O donors in Lagos. Niger J Clin Pract. 2015;18:328–32.
Özgönenel B, Kukreja G, O’Malley B, Bluth MH. Neonatal BO incompatibility is associated with a positive cord blood direct antiglobulin test in infants of black ethnicity. J Pediatr Hematol Oncol. 2015;37:e453–457.
Ozolek JA, Watchko JF, Mimouni F. Prevalence and lack of clinical significance of blood group incompatibility in mothers with blood type A or B. J Pediatr. 1994;125:87–91.
Christensen RD, Yaish HM. Hemolytic disorders causing severe neonatal hyperbilirubinemia. Clin Perinatol. 2015;42:515–22.
Yaish HM, Christensen RD, Lemons RS. Neonatal non-immune hemolytic anemia. Curr Opin Pediatr. 2017;29:12–18.
Acknowledgements
We thank Dr. Jon F. Watchko, Professor of Pediatrics and of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine, for his helpful suggestions.
Funding
None. The time-allocation for study participation of all authors was as part of their research assignment from their employer (University of Utah or Intermountain Healthcare).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Christensen, R.D., Baer, V.L., MacQueen, B.C. et al. ABO hemolytic disease of the fetus and newborn: thirteen years of data after implementing a universal bilirubin screening and management program. J Perinatol 38, 517–525 (2018). https://doi.org/10.1038/s41372-018-0048-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0048-4
This article is cited by
-
Alloimmune hemolytic disease of the fetus and newborn: genetics, structure, and function of the commonly involved erythrocyte antigens
Journal of Perinatology (2023)
-
ABO hemolytic disease of the newborn: a need for clarity and consistency in diagnosis
Journal of Perinatology (2023)
-
Absence of severe neonatal ABO hemolytic disease at Intermountain Healthcare. Why?
Journal of Perinatology (2020)
-
TcB, FFR, phototherapy and the persistent occurrence of kernicterus spectrum disorder
Journal of Perinatology (2020)