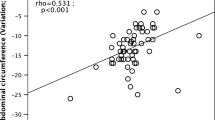
Abstract
We investigated the effect of pharmacologically induced weight loss on markers of glucagon resistance in individuals with overweight during treatment with the glucagon-like peptide-1 receptor agonist liraglutide. We performed an open-label study in 14 men with overweight (age 38 ± 11 years, BMI 32 ± 4 kg/m2) without simultaneously diabetes. Subjects were treated with liraglutide, initiated and titrated with 0.6 mg/day/week to reach the final dose of 3.0 mg/day. Subjects were examined at baseline, during titration (Week 4), after 2 weeks of steady state (Week 6) of final dosing of liraglutide and 3 weeks after discontinuation of liraglutide (follow-up). Study participants lost 3.3 ± 1.9 kg (3%) total body weight during the first 4 weeks of treatment with liraglutide. Simultaneously, liver fat content decreased from 12.4 ± 11.6% to 10.2 ± 11.1%, p = 0.025, whereas fat content in the spleen and subcutaneous tissue was unaltered. Markers of glucagon resistance, including plasma glucagon and the glucagon-alanine-index, also decreased significantly during treatment, but total and individual plasma amino acid concentrations did not. Insulin resistance (HOMA-IR) was unchanged during treatment, whereas insulin clearance increased. Treatment with the GLP-1 receptor analogue liraglutide decreased liver fat content, and simultaneously attenuated glucagon concentrations and the glucagon-alanine index in individuals with overweight without diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Wewer Albrechtsen NJ, Kuhre RE, Pedersen J, Knop FK, Holst JJ. The biology of glucagon and the consequences of hyperglucagonemia. Biomark Med. 2016;10:1141–51.
Solloway MJ, Madjidi A, Gu C, Lowe JB, Peterson AS, Allan BB, et al. Glucagon Couples Hepatic Amino Acid Catabolism to mTOR-Dependent Regulation of a -Cell Mass Article Glucagon Couples Hepatic Amino Acid Catabolism to mTOR-Dependent Regulation of a -Cell Mass. Cell Rep. 2015;1–16. https://pubmed.ncbi.nlm.nih.gov/26166562/.
Holst JJ, Wewer Albrechtsen NJ, Pedersen J, Knop FK. Glucagon and Amino Acids Are Linked in a Mutual Feedback Cycle: The Liver-α-Cell Axis. Diabetes. 2017;66:235–40.
Winther-Sørensen M, Galsgaard KD, Santos A, Trammell SAJ, Sulek K, Kuhre RE, et al. Glucagon acutely regulates hepatic amino acid catabolism and the effect may be disturbed by steatosis. Mol Metab. 2020;42:101080.
Galsgaard KD, Jepsen SL, Kjeldsen SAS, Pedersen J, Albrechtsen NJW, Holst JJ. Alanine, arginine, cysteine, and proline, but not glutamine, are substrates for, and acute mediators of, the liver-α-cell axis in female mice. Am J Physiol - Endocrinol Metab. 2020;318:E920–9.
Wewer Albrechtsen NJ, Junker AE, Christensen M, Hædersdal S, Wibrand F, Lund AM, et al. Hyperglucagonemia correlates with plasma levels of non-branched-chain amino acids in patients with liver disease independent of type 2 diabetes. Am J Physiol - Gastrointest Liver Physiol. 2018;314:G91–6.
Suppli MP, Bagger JI, Lund A, Demant M, van Hall G, Strandberg C, et al. Glucagon resistance at the level of amino acid turnover in obese subjects with hepatic steatosis. Diabetes. 2020;69:1090–9.
Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al. The Liver-α-Cell Axis and Type 2 Diabetes. Endocr Rev. 2019;40:1353–66.
Wewer Albrechtsen NJ, Færch K, Jensen TM, Witte DR, Pedersen J, Mahendran Y, et al. Evidence of a liver–alpha cell axis in humans: hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Diabetologia. 2018;61:671–80.
Gar C, Haschka SJ, Kern-Matschilles S, Rauch B, Sacco V, Prehn C, et al. The liver-alpha cell axis associates with liver fat and insulin resistance: a validation study in women with non-steatotic liver fat levels. Diabetologia. 2021;64:512–20.
Pedersen JS, Rygg MO, Kristiansen VB, Olsen BH, Serizawa RR, Holst JJ, et al. Nonalcoholic Fatty Liver Disease Impairs the Liver–Alpha Cell Axis Independent of Hepatic Inflammation and Fibrosis. Hepatol Commun. 2020;4:1610–23.
Svane MS, Johannesen HH, Martinussen C, Bojsen-Møller KN, Hansen ML, Hansen AE, et al. No effects of a 6-week intervention with a glucagon-like peptide-1 receptor agonist on pancreatic volume and oedema in obese men without diabetes. Diabetes, Obes Metab. 2020;22:1837–46.
Wewer Albrechtsen NJ, Kjeldsen SAS, Jensen NJ, Rungby J, Veedfald S, Bojsen-Møller KN, et al. On measurements of glucagon secretion in healthy, obese, and Roux-en-Y gastric bypass operated individuals using sandwich ELISA. Scand J Clin Lab Investig. 2021;1–9. https://pubmed.ncbi.nlm.nih.gov/34935574/.
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Investig. 1993;91:301–7.
Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Targher G. Glucagon-like peptide-1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: An updated meta-analysis of randomized controlled trials. Metabolites. 2021;11:1–13.
Acknowledgements
The authors acknowledge Christine Rasmussen and Fie Johanne Andreasen (Department of Clinical Biochemistry, Rigshospitalet) for technical assistance. Furthermore, we thank Annette Witt (Department of Endocrinology, Hvidovre) for logistics and assistance with titration of study medicine and finally we thank Birgitte Vilsbøll/Lotte Lund from Good Clinical Practice (GCP-Unit, University of Copenhagen) for discussions and inputs on the protocol. Finally, we thank Prof. Liselotte Højgaard (Department of Clinical Physiology and Nuclear Medicine, Rigshospitalet) and Dr. Linda Hilsted (Department of Clinical Biochemistry, Rigshospitalet) for their support.
Funding
The study was supported by unrestricted grants from the Novo Nordisk Foundation and Rigshospitalet. NJWA is supported by NNF Excellence Emerging Investigator Grant – Endocrinology and Metabolism (Application No. NNF19OC0055001), EFSD Future Leader Award (NNF21SA0072746) and DFF Sapere Aude (1052-00003B). Novo Nordisk Foundation Center for Protein Research is supported financially by the Novo Nordisk Foundation (Grant agreement NNF14CC0001). The funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Author information
Authors and Affiliations
Contributions
MSS, HHJ and NJWA conception of research; MSS, HHJ and NJWA design of research; MSS, CM, and SM recruited study participants; MSS, and CM conducted the clinical visits and monitored treatment; HHJ, MLH, AEH, SHK, TLK, AL, AK, and JL designed and performed MR imaging; NJWA performed and analyzed biochemical analyses; MSS, and NJWA performed statistical analyses and interpreted results of experiments; MSS prepared figures; MSS, and NJWA drafted manuscript and HHJ, CM, KNB-M, MLH, AEH, CFD, SHK, TLK, AL, AK, SM, JL and JJH edited and revised manuscript; All authors approved final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
NJWA has received speaker fees from MSD and Mercodia and research support from Novo Nordisk A/S, Janssen Pharmaceuticals and Mercodia.CFD has received consultancy/lecture fees from Boehringer Ingelheim, Lilly, Merck/MSD, Novo Nordisk and Novartis. SM has received research grants from Novo Nordisk and Boehringer Ingelheim; lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk and Sanofi Aventis; and is a member of advisory boards of AstraZeneca, Boehringer Ingelheim, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Merck Sharp & Dohme, Novartis, Novo Nordisk and Sanofi Aventis. JJH has served on advisory panels for GlaxoSmithKline, Novo Nordisk, Zealand Pharma, AstraZeneca, MSD, Intarcia and Hanmi and acts as a consultant for Novo Nordisk, and has received research support from Merck, Sharp & Dohme. All other authors have nothing to disclosure.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Svane, M.S., Johannesen, H.H., Hansen, A.E. et al. Four weeks treatment with the GLP-1 receptor analogue liraglutide lowers liver fat and concomitantly circulating glucagon in individuals with overweight. Int J Obes 46, 2058–2062 (2022). https://doi.org/10.1038/s41366-022-01207-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01207-y