Abstract
The small intestine is among the fastest self-renewing tissues in adult mammals. This rapid turnover is fueled by the intestinal stem cells residing in the intestinal crypt. Wnt signaling plays a pivotal role in regulating intestinal stem cell renewal and differentiation, and the dysregulation of this pathway leads to cancer formation. Several studies demonstrate that intestinal stem cells follow neutral drift dynamics, as they divide symmetrically to generate other equipotent stem cells. Competition for niche space and extrinsic signals in the intestinal crypt is the governing mechanism that regulates stemness versus cell differentiation, but the underlying molecular mechanisms are still poorly understood, and it is not yet clear how this process changes during disease. In this review, we highlight the mechanisms that regulate stem cell homeostasis in the small intestine, focusing on Wnt signaling and its regulation by RNF43 and ZNRF3, key inhibitors of the Wnt pathway. Furthermore, we summarize the evidence supporting the current model of intestinal stem cell regulation, highlighting the principles of neutral drift at the basis of intestinal stem cell homeostasis. Finally, we discuss recent studies showing how cancer cells bypass this mechanism to gain a competitive advantage against neighboring normal cells.
Similar content being viewed by others
Introduction to the intestinal epithelium: A “conveyor belt” of different cell types
Many of our organs and tissues require constant cellular turnover throughout life to remain functional. This continuous self-renewal is fueled by dedicated multipotent stem cells, which have a life-long ability to divide and generate the different cell types that comprise specific adult tissues. In mammals, the small intestine is the fastest self-renewing tissue, with its entire epithelium being completely replenished every 3–5 days1,2, thus representing an ideal system for studying the biology of adult stem cells and their specialized niche. Because of the harsh conditions of the intestinal lumen, millions of cells die every day and are rapidly replaced by newly generated differentiated cells originating from actively dividing stem cells in the crypt1. This life-long renewal ability allows the intestinal epithelium to retain its digestive and absorptive functions and to maintain a barrier against infectious microorganisms or toxic substances without being severely compromised.
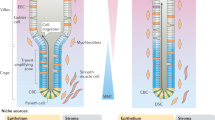
Cells of the small intestine are organized into a simple columnar epithelium that folds into long finger-like structures called villi and projects into the intestinal lumen (Fig. 1). In mice, this basic architecture is established between embryonic Day E13.5 and E18.5, when the pseudostratified gut endoderm transitions into the epithelial monolayer3,4. Once matured postnatally, functional compartmentalization is completed: specialized cells are located in the villi, while proliferating progenitor and stem cells occupy the crypts of Lieberkühn (hereafter simply referred to as ‘crypts’)5,6. Intestinal stem cells (ISCs) reside at the base of each crypt and are also known as crypt base columnar (CBC) cells. These stem cells are readily distinguishable by their slender, wedge-like appearance and scant cytoplasm. Upon cell division, CBC cells produce highly proliferative short-lived progenitors known as transit amplifying (TA) cells, which are located higher in the crypt. TA cells undergo multiple rounds of rapid cell division while differentiating into several cell types7. Differentiating progenitor cells migrate upward along the crypt-to-villus axis (similar to a cellular “conveyor belt”) to produce terminally differentiated cell types in the villus (Fig. 1): enterocytes (belonging to the absorptive lineage), whose function is to complete the breakdown of partly digested food and absorb nutrients; and cells of the secretory lineage, which include mucus-secreting goblet cells; enteroendocrine cells, which produce hormones; Paneth cells, which secrete antimicrobial agents, such as cryptdins and lysozyme; and other minor cell types (Tuft cells and Peyer’s patch-associated M-cells). Paneth cells, unlike the other cell types, migrate downward toward the crypt base, where they intercalate with the CBC stem cells. Upon reaching the tip of the villus, cells ultimately die by anoikis, an anchorage-dependent form of cell death7,8, and are shed into the gut lumen.
Crypts of Lieberkühn surround intestinal villi (six or more crypts per villus) and replenish them with newly formed cells. Crypt base columnar (CBC) stem cells, located at the base of each crypt, represent the driving force that maintains intestine homeostatic renewal. CBC cells divide and originate transit-amplifying (TA) cells located in the upper zone of the crypt, which undergo multiple rounds of mitosis before differentiating into any of the cells of either secretory or absorptive lineages (right panel). CBC cells are intermingled with Paneth cells, specialized secretory cells that support CBC cells by providing growth factors. The balance between self-renewal and differentiation is regulated by morphogenetic signals, including BMP and Wnt. Differentiated cells move to the top of the villus, where they are shed into the intestinal lumen and die by anoikis at the end of their life cycle.
Intestinal stem cells: From homeostasis to regeneration
As noted above, the bases of intestinal crypts harbor actively dividing CBC stem cells. During development, CBC cells derive from the proliferating cells that populate the intervillus regions, although the high level of plasticity allows even nonproliferating cells of the nascent villi to contribute to the adult intestinal stem cells, in part due to the intense remodeling of the fetal intestinal architecture9. CBC cells divide at least once every 24 h and were described as potential intestinal stem cells several decades ago based on fate-mapping experiments using tritiated [3H]thymidine10,11. The formal confirmation that CBC cells are indeed the actual adult intestinal stem cells only came more recently, when specific genetic markers were used for genetic lineage tracing (see below). One of these markers is Lgr5 (Leucin-rich repeat containing G-protein coupled receptor 5), which is specifically expressed in the crypt base, coinciding with CBC cells12.
The identification of stem cell-specific markers opened the possibility for elegant lineage tracing experiments. Lineage tracing is a fate-mapping tool13 that involves introducing a genetically encoded, heritable mark into a putative stem cell. This mark is inherited by the progeny of initially labeled stem cells, allowing their tracking and characterization. Using the Lgr5-eGFP-IRES-CreERT2;R26R-LacZ knock-in mouse model, it was possible to visualize not only the CBC stem cells with green fluorescence but also their direct descendants with β-galactosidase (LacZ) expression triggered by the stem cell-specific activation of tamoxifen-inducible CreERT2 recombinase: ribbons of cells emanating from the crypt bottoms were observed running along the sides of the crypts and comprised the entire spectrum of differentiated intestinal epithelial cells12. Furthermore, labeled cells were still produced over long periods of time, indicating the self-renewal ability of the initially labeled CBC cells. Thus, Lgr5+ CBC cells meet the essential requirements to be considered tissue-resident adult stem cells: multipotency and long-term self-renewal. In addition to Lgr5, other intestinal stem cell-specific markers have been identified, including olfactomedin 4 (Olfm4), Troy (also known as Tnfrsf19), Ascl2, Smoc2, Rnf43, and Znrf314,15,16,17,18,19,20.
Further proof of CBC stemness potential came from ex vivo culture experiments on Lgr5-eGFP reporter mice using single Lgr5+ cells isolated by fluorescence-activated cell sorting (FACS). The isolated cells could generate 3D self-renewing and self-organizing epithelial structures, denoted as ‘organoids’, which recapitulated the architecture and cellular composition of the adult intestine21. Intestinal organoids can form in the absence of mesenchymal cells and require a limited set of growth factors, namely, EGF, Noggin, and R-spondin21. Of note, Lgr5+ stem cells (or cells expressing Lgr6, another paralog) were identified in several other tissues, including the colon, hair follicle, stomach pylorus and corpus, mammary glands, ovarian surface epithelium, and more recently, skeletal muscle12,22,23,24,25,26,27. The adult stem cell and organoid research fields have developed considerably after the initial success of mouse intestinal organoid culture, holding great promise as novel in vitro models for studying organogenesis and organ maintenance as well as related human diseases28,29,30,31,32.
In addition to crypt base stem cells, for a long time, it has been speculated that alternative intestinal stem cells could reside in position +4 (with respect to the crypt base), where rare DNA label-retaining cells (LRC) were found33,34,35,36. DNA label retention is a feature of slowly cycling cells, which remain in a quiescent state37. These cells are thought to be different from Lgr5+ stem cells, as they do not actively divide in homeostatic conditions but re-enter the cell cycle following tissue damage that depletes Lgr5+ cells, driving the regenerative process that reconstitutes the injured tissue. Several genetic markers have been reported as enriched in position +4, such as Bmi138,39, Tert40, Hopx41, and Lrig142, although the specificity of these markers has been debated16, as described below. Using diphteria toxin receptor (DTR) mouse genetic models that allow the selective ablation of CBC cells, non-Lgr5+ progenitor cells were shown to act as a reserve stem cell pool that could regenerate all intestinal cell types, including Lgr5+ stem cells43. Notably, the intestinal architecture was maintained even after prolonged damage, suggesting that Lgr5+ cells are in theory dispensable for tissue maintenance. However, the specificity of the +4 markers has been questioned after the finding that Lgr5+ crypt base stem cells also express robust levels of these genes16. In-depth analysis of the Lgr5+ cell transcriptome and proteome provided a combined stem cell signature of approximately 500 genes and proteins enriched in Lgr5+ ISCs, which included putative +4 markers16. Single-molecule fluorescence in situ revealed that +4 markers are not confined to a distinct stem cell population; rather, they show broad expression profiles throughout the crypt that overlap with Lgr5+ stem cells44. More recently, single-cell RNA sequencing (scRNA-seq) analyses have led to similar conclusions, showing that several of these genes, including Bmi1, are expressed in multiple cell clusters at similar levels45,46. Taken together, these findings suggest the presence of progenitor cell populations with strong plasticity, but the identification of their marker genes has not been fully established.
Several other studies have highlighted the shared plasticity among differentiating progenitors both in secretory and absorptive lineages in the intestinal epithelium. Within the secretory lineage, Delta-like 1+ (Dll1+) cells give rise to all secretory cell types (enteroendocrine, tuft, goblet, and Paneth) during homeostasis47, indicating that these rare Dll1+ cells are indeed secretory progenitors. Lineage tracing experiments coupled with irradiation to kill actively cycling Lgr5+ cells showed that Dll1+ secretory progenitors can revert to Lgr5+ cells and form long-lived clones along the villus axis, consisting of both absorptive and secretory cells47. Similar observations were also reported by using an elegant genetic labeling strategy to mark the LRCs in the crypt. This work demonstrated that the LRCs in the crypt are normally secretory progenitors, which can show a broader clonogenic potential by dedifferentiating and regaining multipotent stem cell features after tissue injury48. In addition, using the Alpi-IRES-CreERT2 knock-in allele, early enterocyte progenitors were also found to have plasticity comparable to that of their secretory counterparts when Lgr5+ stem cells were specifically depleted49. Due to their abundance, it seems that enterocyte progenitors are able to form a larger reservoir of stem cells during crypt regeneration. However, enterocyte progenitors are also actively cycling, so irradiation would deplete them as well as Lgr5+ stem cells, leaving the slow-cycling secretory progenitors to hold a key role in tissue regeneration. Therefore, depending on the types of injury, the two lineage progenitors will be actively and selectively adopted for the re-establishment of the intestinal stem cell pool.
Interestingly, achaete-scute-like 2 (Ascl2) has been recently identified as a key factor for the process of intestinal epithelial cell dedifferentiation45. Ascl2 is a basic helix-loop-helix transcription factor whose expression is under Wnt regulation in the intestinal crypt and is required for stem cell survival and identity15,20. Upon treatment with tamoxifen, ISC descendants of Lgr5GFP-CreER(T2); R26RtdTom mice were permanently labeled with a red fluorescent marker (tdTomato). After crypt stem cells were eliminated through radiation, intestinal crypts were soon repopulated by stem cells expressing not only GFP (under the control of Lgr5 promoter) but also tdTomato, indicating that these new stem cells originated from their recent progeny, e.g., cells that were on their way to differentiation45. Ascl2 was ectopically expressed in cells well above position +5 and was absolutely required for the recovery of ISCs upon damage, probably by the activation of a regenerative transcriptional program including interleukin receptor Il11ra1 among its targets. Additionally, this study showed that not only secretory progenitors but also absorptive lineages were able to recover lost ISCs, as observed in earlier work49.
More recently, an injury-responsive stem cell population has been identified, expressing high levels of the stress-response gene Clusterin (Clu+)46. These Clu+ cells show characteristics of a fetal gene signature, and their appearance depends on active Yap1, a transcription factor inhibited by Hippo signaling. Under different types of damage, Clu+ cells were able to repopulate CBC-depleted crypts, reconstituting all the main intestinal cells, including CBC cells, in a hierarchical manner46. Nevertheless, whether Clu+ cells derive from preexisting Clu+ cells or from ISCs and other progenitors through reprogramming in response to injury remains ambiguous. Notably, Yap has been found to induce the reprogramming of colonic epithelial and stem cells following lesions, generating a population of Klf6+ cells characterized by a wound-healing gene signature and low Wnt activity50. While several works have shown that Yap is required for intestinal regeneration50,51,52, it is unclear whether all the cells participating in regeneration, including the abovementioned absorptive and secretory lineages, undergo Yap-driven reprogramming.
In conclusion, Lgr5+ CBC cells represent the workhorse that sustains the life-long homeostatic self-renewal of the intestine. On the other hand, after epithelial tissue damage, various cell types participate in the regeneration process depending on the contexts and available populations that remain intact in the tissue (see Table 1). How all these identified cell types are orchestrated to perform efficient tissue repair is incompletely characterized, but the players and signaling pathways involved are being rapidly revealed, leading to a clearer understating of not only normal regeneration but also chronic tissue damage.
A brief overview of Wnt/β-catenin signaling
Several conserved signaling pathways, including Notch, transforming growth factor-β (TGF-β), epidermal growth factor (EGF), bone morphogenetic protein (BMP), Sonic hedgehog (SHH), and Wnt, operate in the crypt niche. The orchestration of these different pathways determines the proper balance between the proliferation, quiescence, fate decision, and differentiation of intestinal stem cells and their progeny6. Active Notch and EGF signaling, for example, are known to maintain an undifferentiated status and control the number of stem cells in the crypt, while BMP signaling drives their differentiation31,32. Among these different signals, however, the canonical Wnt/β-catenin pathway plays a pivotal role in regulating intestinal stem cell homeostasis.
Wnt ligands belong to a conserved family of secreted glycoproteins modified by palmitoylation on a conserved serine residue53,54,55,56. Canonical Wnt signaling ultimately depends on the activity of the transcriptional coactivator β-catenin as the main effector protein of the TCF/β-catenin complex. In the absence of Wnt ligands, a cytoplasmic ‘destruction’ complex comprising the tumor suppressors adenomatous polyposis coli (APC), Axin, casein kinase 1 (CK1) and glycogen synthase kinase 3 (Gsk3) binds and promotes the phosphorylation of β-catenin for proteasomal degradation, keeping the Wnt pathway in the OFF state57. Conversely, when Wnt ligands bind to their cognate receptors Frizzled (Fzd) and low-density lipoprotein receptor-related protein 5/6 (Lrp5/6), several effectors, including Axin and Disheveled (Dvl), are recruited to the plasma membrane, forming a multiprotein complex known as the signalosome that serves as a platform for downstream effector protein interactions58,59. Multiple events, such as the conformational inactivation of Axin, inhibition of β-transducin repeat containing E3 (β-TrCP)-mediated β-catenin ubiquitination and sequestration of negative Wnt regulators into multivesicular endosomes concur to inhibit β-catenin degradation59,60,61,62,63,64. Consequently, stabilized β-catenin shuttles to the nucleus, where it binds the DNA-associated protein T-cell factor/lymphoid enhancer factor (TCF/LEF) and promotes the transcription of downstream Wnt target genes (Fig. 2).
Binding of Wnt ligands to cognate receptors Frizzled and Lrp5/6 leads to the formation of a multiprotein complex known as the signalosome. The signalosome recruits Dvl, Axin, Gsk3, and casein kinases to the plasma membrane, leading to the dissociation of the cytosolic destruction complex. In turn, this promotes β-catenin stabilization, which translocates into the nucleus. Here, together with TCF/LEF transcription factors, β-catenin induces Wnt target gene activation. Axin2, Rnf43 and Znrf3 are both target genes and inhibitors of the Wnt pathway, thus forming a negative feedback loop.
Wnt ligands can transmit signals without β-catenin stabilization, which is denoted as noncanonical Wnt signaling. The noncanonical Wnt pathways are highly diverse, and new branches are continually added. The most well-defined of these pathways are planar cell polarity (PCP) and intracellular calcium signaling, which play critical roles in developmental and physiological processes65. Canonical and noncanonical Wnt pathways share several signaling components and retain antagonistic roles to one another, implying the need to maintain a balance between the two66. Indeed, recent studies proposed the Wnt/PCP pathway as a new niche signal controlling ISC lineage selection, priming stem cells to adopt Paneth or other secretory cell fates, and intestinal regeneration67,68. Understanding the complex network of noncanonical and canonical Wnt pathways could lead to more delicate Wnt signaling manipulation, potentially paving the way for a breakthrough in related disorders.
Wnt/β-catenin is required for ISC self-renewal and stemness
In the intestine, Wnt/β-catenin activity is highest at the bottom of the crypt, as can be indicated by nuclear β-catenin immunostaining or knock-in Wnt-reporter mouse lines69. Previous work suggested that Wnt/β-catenin is fundamental for intestinal crypt formation during the late stages of fetal development. The deletion of TCF-4 (encoded by the tcf7l2 gene) in mice disrupts the formation of crypt regions with a complete loss of cycling cells from the intervillus regions at Day E18.5, which are instead replaced by differentiated cells70. In fact, together with β-catenin, TCF-4 controls a genetic program that promotes the proliferation and undifferentiated status of intestinal cells, in part by upregulating c-Myc (required for cell division) and downregulating p21, which would otherwise induce cell cycle arrest and differentiation71. Importantly, TCF-4 maintains a similar role during ISC homeostasis in adult mice, as CreERT2-mediated conditional deletion in Tcf4LoxP/LoxP mice caused a dramatic reduction in the number of proliferating cells in the crypt as well as in the expression of Wnt targets CD44 and Sox972. Olfm4-expressing CBC cells were soon lost after Cre induction, probably because of extensive cell death, followed by a collapse of the crypt-villus structure. Notably, other members of the TCF family, such as TCF-1 or TCF-3/Tcf7l1, were shown to be dispensable in regulating intestinal homeostasis, indicating a specific function for TCF-472. TCF-4 deletion affects the proliferation and survival of not only stem cells but also Paneth cells, which decline rapidly after Cre induction72. Comparable to TCF observations, a β-catenin conditional knockout (KO) also resulted in the loss of ISCs and premature differentiation in the adult mouse intestine73.
A requirement for secreted Wnt ligands in maintaining ISC homeostasis was indirectly attested by using transgenic mice that overexpressed Dkk1, a well-known extracellular inhibitor specific for canonical Wnt ligands74,75. Dkk1 overexpression disrupted intestinal organization and ISC proliferation76, similar to TCF-4 or the β-catenin KO. More recently, the importance of Wnt ligands in the intestine has been confirmed using selective chemical inhibitors of Porcupine, an endoplasmic reticulum (ER) membrane-bound O-acyltransferase that is required for Wnt palmitoylation and secretion. Systemic treatments of mice with the Porcupine inhibitors C59 or LGK974 have been shown to greatly downregulate stem cell markers and Wnt target genes and, at high dosages, prevent CBC proliferation and homeostatic self-renewal in the small intestine77,78. Several Wnt ligands act redundantly in the crypt to maintain ISC homeostasis77,79 and are produced from both epithelial and mesenchymal sources, with Wnt3 being expressed specifically in Paneth cells80,81. Using an HA-tagged Wnt3 mouse knock-in line (Wnt3HA/HA), it was possible to visualize the endogenous Wnt ligands in the intestine for the first time. Wnt3 showed minimal diffusion in the crypt, forming a short-range signaling gradient82. Interestingly, Wnt3 did not diffuse passively but was instead transferred from Paneth cells (which produce the ligand) to the closest neighboring CBC cells, where it remained tethered to the cell surface through binding to Frizzled. The surface-bound Wnt3 was then disseminated by stem-cell division, generating a gradient of Wnt proteins along the crypt-villus axis82.
The power of mouse genetics has been fundamental to further dissecting the role of Wnt signaling components during ISC homeostasis. For instance, Ras-like proto-oncogenes A and B (RalA and RalB), two GTPases involved in Wnt signalosome endocytic activation, were recently shown to be required for CBC maintenance in small intestinal crypts83. The conditional KO of the two isoforms, both expressed in the intestinal crypt, resulted in strong reductions in the stem cell markers Lgr5 and Olfm4, with a concomitant decrease in nuclear β-catenin staining in the crypt bottom. Furthermore, single Lgr5+ cells isolated from RalA or RalB KO crypts were inefficient in forming organoids, indicating a loss of stemness83. In addition to the expression of specific components of the Wnt machinery, other factors, such as physical properties, may contribute to high levels of Wnt signaling. Indeed, a correlation between the compressed cell volume of Lgr5+ ISCs and active Wnt/β-catenin signaling has recently been proposed84. Among the cellular compartments in the intestinal epithelium, Lgr5+ ISCs with greater levels of nuclear β-catenin have a smaller and thinner shape, implying a higher level of physical stress. Interestingly, artificial cell compression enhanced Wnt/β-catenin signaling activity independent of β-catenin release from the cadherin-catenin association, but in a Wnt ligand-dependent manner. Consistently, osmotic and mechanical compression promoted organoid formation from isolated crypts, which often exhibited a cystic, more undifferentiated state, enriching the Lgr5+ ISC population and their self-renewal activity84. Mechanistically, volumetric compression favors the molecular crowding and stabilization of the Wnt signalosome, which may occur on the basis of higher Wnt responsiveness in ISCs84. However, direct in vivo evidence is still missing.
Genetic mutations that cause the unrestrained activation of Wnt/β-catenin signaling induce strong overproliferation and are the cause of many forms of colorectal cancer (CRC). In fact, it is estimated that over 90% of sporadic CRCs contain at least one mutation in a known Wnt regulator85. For example, truncating mutations in APC or AXIN2 or mutations that stabilize β-catenin all lead to high levels of nuclear β-catenin/TCF complexes and Wnt activation in intestinal cancers86,87,88. APC alterations are found in 80% of human colorectal tumors, while CTNNB1 (the human homolog encoding β-catenin) and TCF7L2 are mutated only in 5 and 9% of CRCs, respectively85. Wnt activating mutations have also been described in regard to other cancers89, confirming that Wnt signaling is a major driver in tumor initiation in a variety of organs. APC mutation occurs early in premalignant (benign) lesions of the intestine, such as small polyps90, and APCmin (multiple intestinal neoplasia) mice carrying a dominant mutation in APC are predisposed to develop multiple intestinal adenomas91,92, similar to mutations causing familial adenomatous polyposis (FAP) in humans. The progression from benign adenoma to malignant carcinoma requires the sequential acquisition of additional specific genetic alterations93, an event that can be modeled using CRISPR/Cas9 genetically modified intestinal organoids94,95. APC mutations are commonly found to activate Wnt/β-catenin signaling and initiate the formation of benign polyps, while the progression to CRC requires activating mutations in the EGF pathway and inactivating mutations in P53 and TGF-β. However, the restoration of APC is sufficient to sustain cell differentiation, homeostatic proliferation, and tumor regression in CRC with multiple genetic mutations96, suggesting that even advanced cancers are still dependent on the constitutive activation of Wnt. Altogether, these data indicate that Wnt/β-catenin signaling is required for ISC proliferation and maintenance, while its abnormal activation is a major cause of tumor formation.
Rnf43 and Znrf3 are fundamental feedback inhibitors of Wnt signaling in the intestinal crypt
Due to its strong mitogenic activity, several mechanisms have evolved to maintain Wnt/β-catenin signaling under tight control. Multiple inhibitors operating at different levels of the Wnt/β-catenin signaling cascade, including Axin97 and SH3 domain-binding protein 4 (SH3BP4)98, contribute to maintaining intestinal homeostasis. However, it seems that the main feedback mechanism ensuring adequate levels of Wnt signaling in the intestinal crypt occurs at the plasma membrane. In 2012, a screening of Wnt target genes expressed in the intestine revealed that two closely related single-pass transmembrane E3 ubiquitin ligases, Rnf43 and Znrf3 (R/Z), are specifically expressed in crypt stem cells in response to Wnt/β-catenin signaling17. Importantly, it was shown that R/Z dampens Wnt signaling through the ubiquitination of Frizzled and Lrp5/6 receptors, which are then degraded through the endolysosomal pathway (Fig. 3)17,99. Of note, Dvl is required for the R/Z-mediated degradation of Wnt receptors100. Hence, R/Z acts as a Wnt feedback inhibitor at the receptor level. The KO of these genes in the mouse intestine elicited the dramatic proliferation and expansion of crypt cells, with the formation of adenomas similar to the loss of APC, thus revealing their tumor suppressor nature17. Nonetheless, unlike APC mutations, R/Z-deficient cells still depend on a source of paracrine Wnt ligand101.
Rnf43/Znrf3 (R/Z) promotes Frizzled and Lrp5/6 ubiquitination, assisted by the cytosolic adaptor Dvl. Once ubiquitinated, Wnt receptors are internalized by endocytosis and degraded via the lysosomal system. R/Z activity is regulated by the phosphorylation of the cytosolic tail by casein kinase 1 (CK1). The secreted Wnt agonist Rspo inhibits R/Z by forming a ternary complex with Lgr4/5/6 receptors, which in turn triggers the autoubiquitination of R/Z followed by the endocytosis of the complex. The deubiquitinase Usp42 stabilizes R/Z at the plasma membrane by removing ubiquitin from R/Z proteins, hence inhibiting Wnt signaling.
Notably, R/Z is the target of stem cell-specific Lgr receptors, and several studies have contributed to elucidating the underlying mechanistic regulation between R/Z and Lgr. Lgr4/5/6 are members of a subfamily of G-protein coupled receptors, characterized by a large extracellular domain containing 16–18 leucine-rich repeats and expressed in several types of stem cells102. Importantly, the Lgr leucin-rich domain interacts with R-spondins, a long-known class of 4 secreted Wnt agonists (Rspo 1–4)103,104,105,106,107. When Rspo binds to Lgr4/5/6, they promote the formation of a trimeric complex containing R/Z, followed by clearance of the E3 ligases from the plasma membrane as a result of autoubiquitination (Fig. 3)99,108. In this way, the Wnt receptors become stabilized, and cells are sensitized (i.e., respond more robustly) to Wnt ligands. Accordingly, Rspo/Lgr have no signaling activity per se but sustain a robust Wnt signal. Together with R/Z, both Lgr and Rspo play a fundamental role in regulating stem cell activity in the intestinal crypt through the modulation of Wnt signaling. The conditional KO of Lgr5 and its close homolog Lgr4 caused a rapid loss of stem cells and a strong reduction in CBC marker expression107. Conversely, the overexpression of Rspo is known to induce the strong proliferation and expansion of the stem cell compartment in the small intestine, leading to the formation of adenomas in a similar way to R/Z depletion109,110,111. Since Wnt ligand diffusion in the crypt requires tethering on stem cell membranes through Fzd, the interplay between R/Z and the Rspo/Lgr signaling system plays an active role in determining the shape and size of the Wnt signaling gradient through the modulation of Wnt receptor turnover82. Unsurprisingly, RNF43 mutations or RSPO2/3 chromosomal translocations, both of which boost Wnt signaling, are associated with different forms of colorectal tumors (with mutation frequencies of 18 and 3–8%, respectively108,112), as well as pancreatic and gastric cancers112,113,114,115,116,117,118,119,120. These mutations are mutually exclusive with APC mutations112,117, and in contrast to the latter, they are all characterized by their dependency on a source of Wnt ligand (Wnt addiction). Thus, Rspo/Lgr/RZ are prominent players in regulating the homeostasis of ISCs as well as other tissue-specific adult stem cells102.
Until recently, it has remained unclear how posttranslational modifications regulate R/Z121. CK1-mediated phosphorylation in the serine-rich motif near the Dvl interacting region (DIR) on the C-terminal tail of Rnf43 acts as a “phospho-switch” to increase its Wnt-antagonistic activity122. This region is often mutated or truncated in oncogenic forms of Rnf43, which consequently eliminates the negative regulation of Fzd122. CK1 phosphorylation may also support an alternative Wnt-inhibitory function of Rnf43, requiring interactions with the Wnt destruction complex independent of Fzd turnover123. Certain cancer-associated Rnf43 truncated mutants promote Wnt signaling while still retaining the ability to downregulate Fzd. This is due to a stronger association with Axin and CK1, which trap them at the plasma membrane and cause the dissociation of the destruction complex, activating the Wnt pathway at the level of β-catenin-mediated transcription123. On the other hand, dephosphorylation by the tumor suppressor Ptprk is essential for Znrf3 to promote Fzd downregulation in an endocytosis-dependent fashion124. Thus, it is possible that phosphorylation differentially regulates the two functional homolog E3 ligases.
Finally, it is well established that Lgr regulates R/Z clearance through autoubiquitination99,108, thereby promoting Wnt receptor stabilization. Interestingly, R/Z autoubiquitination is also a dynamic process and is reversed by Usp42, a deubiquitinase that stabilizes R/Z levels at the plasma membrane, counteracting Rspo/Lgr4-mediated clearance and promoting Wnt inhibition125,126. Usp42 mutations are found in colorectal cancer, and Usp42 KO organoids can grow in the absence of Rspo125, similar to R/Z double KO organoids101. The complexity of R/Z modulation has only begun to be unraveled, and the identification of novel factors and mechanisms involved in the E3 ligase regulatory network highlights the importance of these Wnt antagonists in stem cell and cancer biology.
ISCs are regulated by neutral drift dynamics
A long-standing question in the stem cell field is how ISCs are maintained and how the choice between different fates is regulated. A popular model from studies on Drosophila germline stem cells and C. elegans proposes that the asymmetric division of stem cells generates two daughter cells with divergent fates: a new stem cell and a TA progenitor that will differentiate127,128. It has been argued whether ISC homeostasis is also governed by asymmetric division. However, there is no evidence that CBC cells adopt unequal fates based on intrinsic mechanisms. Rather, it seems that the choice between self-renewal and differentiation is stochastic. Elegant lineage-tracing experiments using mice expressing a multicolor fluorescent reporter (denoted as ‘confetti’) (Fig. 4), together with quantitative mathematical models, allowed the analysis of CBC stem cell dynamics129,130. Stem cell clones (expressing one of four different fluorescent proteins) either randomly expanded to eventually colonize the entire crypt or were lost and replaced by other clones (expressing a different fluorescent protein). Surviving clones maintained the pool of Lgr5+ stem cells, while clones comprising only cells undergoing differentiation soon became extinct, losing their fluorescent trace (Fig. 4). By 2 months after the start of lineage tracing, almost all the crypts had become monoclonal (or fixed, that is, each crypt derived from a single stem cell expressing one particular fluorescent protein). Therefore, CBC stem cells are all endowed with the same long-term stemness potential and compete equally (hence, the term ‘neutral competition’) for the available niche space, the fundamental determinant for their ultimate fate. Upon cell division, cells that are pushed out of the niche space will undergo differentiation, while those that remain at the base will maintain their self-renewal capability131. In other words, cells at the base of the crypt have a higher chance of surviving and forming clones that will repopulate the entire crypt, while those in the upper part of the crypt are more likely to be pushed into the TA zone, after which they will undergo differentiation. Thus, intestinal stem cell homeostasis is maintained at the population level, where the loss of stem cells is compensated by the generation of other stem cells. Over time, one stem cell clone will eventually outcompete the others by neutral drift dynamics, substantiating the long-known notion that crypts tend to become monoclonal (Fig. 4)132,133.
Top: the R26R-confetti allele functions as a stochastic multicolor reporter, in which Cre-dependent recombination induces the mutually exclusive expression of one of four different fluorescent proteins (green, yellow, red, and cyan). The specific activation of confetti in intestinal stem cells allows us to follow clonal dynamics in the intestinal crypt, as individual ISCs and their direct progeny express the same unique fluorescent protein. Bottom: an intestinal crypt (visualized from a bottom view) containing ISCs labeled with different fluorescent proteins (red, yellow, and cyan) or unlabeled (light gray). Paneth cells (dark gray) are intercalated between the ISCs. Over time, some clones will expand (see the yellow clone in the example), while others will shrink or disappear completely (cyan and red clones, respectively) as a result of neutral competition.
What factors limit the available niche space? A possible answer is contact with Paneth cells, which produce many of the growth factors required for stem cell maintenance. The genetic depletion of Paneth cells in Sox9fl/fl mice results in stem cell loss, and coculturing Paneth cells with isolated Lgr5+ cells maximizes organoid formation81. Paneth cells intermingle with CBC cells (which are squeezed in between) and organize together into an icosidodecahedron geometry, similar to the tessellation of a soccer ball (Fig. 4). This spatial arrangement maximizes the contact between stem cells and Paneth cell membranes, to which some of the signaling factors required for stem cell renewal are bound, such as Wnt382. Thus, Paneth cells are likely the prime controllers for establishing the ISC niche by producing and possibly confining growth factors.
In addition to Paneth cells, several studies have shown that mesenchymal cells in the stromal niche underlying crypts or the villus tip also play a role in intestinal stem cell maintenance. Different subgroups of rare mesenchymal cells have been identified, such as Gli1-expressing cells134, Foxl1+ telocytes135, Pdgf receptor-α (PdgfRα)+ pericryptal myofibroblasts136, Lgr5+ telocytes137, Ng2+ pericryptal cells138, Gremlin1+ trophocytes139 and Map3k2-regulated stromal cells140. Altogether, these cells provide secreted factors, such as Wnts and R-spondins, indispensable for ISC growth and maintenance in vivo during homeostasis and regeneration and can support Lgr5-derived organoid growth when cocultured in vitro.
Although the complete organization of the ISC niche is still elusive, these data point to a model where Lgr5+ CBC cells compete for stemness factors that are spatially restricted in the confined space of the crypt niche, and the R/Z-Rspo-Lgr axis plays a prominent role in regulating the stem cell response to these factors. Interestingly, similar competition mechanisms may operate in the stem cell compartments of other tissues with sustained turnover, such as the gastric tissue141,142 or the skin143, potentially working as a general mechanism underlying adult stem cell homeostasis.
Winning the clone wars: Cancer cells induce healthy neighbors to join the dark side
It is still incompletely understood how cell competition dynamics work in the context of diseases such as cancer. Following neutral drift dynamics, ISCs can randomly be replaced by any other equipotent ISC present in the crypt. This also means that an ISC that has acquired a specific mutation also has a chance of being replaced by other wild-type (WT) cells. Cell competition is an evolutionarily conserved process that prevents the accumulation of harmful mutations and eliminates unfit cells and thus plays an important role in maintaining tissue health143,144,145. However, what happens when a mutation confers a fitness advantage over neighboring normal cells, as is the case for many oncogenic mutations? The loss of APC in ISCs leads rapidly to the formation of intestinal adenomas in mice146. Mutated ISCs are more efficient in generating tumors than progenitor or differentiated cells, which have a short lifespan and are usually shed off into the intestinal lumen within 3–4 days146. As revealed by previous studies, precancerous ISCs with a KrasG12D activating mutation or APC−/− show a substantial survival advantage over normal stem cells, with a higher probability of crypt fixation due to biased drift147,148. The expansion of mutated cells predisposes a tissue to cancer development and progression, a process known as field cancerization. Nonetheless, the presence of such advantageous genetic alterations does not guarantee immediate success in colonization, as only a relatively low number of APC−/− or KrasG12D stem cells will finally reach crypt fixation147. In fact, most mutated cells can still be eliminated stochastically by surrounding normal cells through competition mechanisms.
What allows genetically altered cells to eventually subjugate normal, healthy cells and win the competition? To investigate this, our lab has recently used a multicolor reporter system denoted as Red2Onco, derived from the original confetti allele, in which the expression of a red fluorescent protein (RFP) was tied to the overexpression of a specific oncogene (Fig. 5)149. This approach makes it possible to trace clones derived from mutant and WT cells in the same tissue, such as the intestine. Mutations leading to the constitutive activation of the Ras and phosphoinositide 3-kinase (PI3K)-AKT pathways are frequently found in several malignancies, including CRC85,150. Interestingly, we found that ISC clones overexpressing Red2Onco KrasG12D or PIK3CAH1047R showed a substantial survival advantage over WT cells, which rapidly led to the fixation of monoclonal red crypts in the small intestine, as expected by biased competition dynamics. Rather unexpected, however, was the finding that mutant crypts could accelerate clonal fixation in proximal, adjacent WT crypts (labeled with either yellow, blue, or green fluorescent proteins)149. This correlated with a decrease in the number of Lgr5+ WT stem cells and an increase in differentiation markers. A comparative transcriptomic analysis of FACS-sorted mutant and WT cells revealed that our Red2Onco models showed a marked increase in BMP signaling due to the higher production of Bmp2/7 from mutant cells, providing an explanation for the loss of stemness and increase in differentiation. Notably, stromal cells from the mesenchymal population also showed an increase in the expression of the Wnt antagonists secreted Frizzled-related protein 2 and 4 (Sfrp2 and 4). This suggests that the oncogene-driven remodeling of the niche environment renders WT cells unfit to compete, favoring the fixation of mutant cells in the intestinal crypts149.
Top: Red2Onco design is based on the original confetti allele. CRISPR/Cas9 genome editing was used to insert a 2 A peptide-Oncogene cassette between the red and cyan fluorescent proteins. This converts the original RFP into a bicistronic construct coexpressing the red fluorescent marker together with an oncogene of choice. Cre recombination occurs as usual, inducing the stochastic expression of one of the four fluorescent reporters. Cells expressing RFP will also express the selected oncogene, while the other colors label wild-type (WT) cells. Bottom: expression of an oncogene, such as KrasG12D or PIK3CAH1047R, confers a survival advantage to red clones, which will rapidly expand into their home crypt. Over time, this will lead to the fixation of the mutant clone into the crypt, which becomes monoclonal red. Due to the cancer cell influence, nearby crypts will also accelerate fixation and become monoclonal (visualized in cyan). Distal crypts are not affected by the red mutant clones. Mutant cells express secreted factors, including prodifferentiation signaling molecules (BMPs) and Wnt antagonists (Notum, Dkk, and Sfrp), which reduce the number of stem cells in proximal WT crypts, driving biased drift. Note that niche remodeling induced by cancer cells also affects nearby stromal cells.
Two additional works have shown similar findings using a different experimental approach. Intestinal organoids derived from APC−/− exhibited growth and survival advantages and rapidly overcame WT organoids in coculture experiments151,152. Of note, APC−/− not only passively outcompeted WT organoids but also actively reduced their clonogenic and expansion capacity. Importantly, the same effect was observed when WT organoids were grown in conditioned medium (CM) derived from mutant cells, clearly indicating a noncell autonomous effect. A transcriptomic profile analysis of APC−/− cells revealed an increase in the expression of several extracellular Wnt inhibitors, such as Wnt inhibitory factor (Wif), Dkk, Sfrp, and Notum151,152, as also shown by in situ hybridizations on APC−/− tumors in vivo. Among the Wnt antagonists, Notum seemed more effective in inhibiting ISC-derived organoid growth and expansion in vitro152, although a cooperative effect with the other antagonists is likely to occur151. Notum is a secreted deacylase that removes palmitoleic acid from Wnts, disengages them from Fzd, and suppresses Wnt signaling153,154. Similar to what was reported by Yum et al.149, Wnt inhibition reduced the expression of transcripts associated with CBC stemness, while the expression of differentiation genes increased151,152. Notably, APC KO cells are shielded from Notum activity, as Notum acts at the ligand–receptor level, while APC loss activates the Wnt pathway downstream of receptor signaling. It was reported that the prevention of Wnt secretion by Porcupine inhibitors favors tumor initiation by reducing the pool of functional stem cells78. Thus, a common scheme emerges from these recent studies, where cancer cells actively reduce the competitive fitness of neighboring WT stem cells by promoting their differentiation, with the consequent loss of their stemness potential. Mutated cells achieve this by utilizing paracrine signaling, e.g., by secreting growth factor inhibitors (e.g., Notum or other Wnt inhibitors) or prodifferentiation factors (such as BMPs), while themselves are “protected” or “surviving” from this suppressive activity149,151,152 (Fig. 5). Rescue experiments further corroborated the specificity of Wnt inhibition in biased competition: the use of Notum chemical inhibitors, Notum genetic knockout, or treatments with the Gsk3 inhibitor LiCl (which activates β-catenin-dependent transcription) were all effective in reducing crypt fixation by APC−/− cells by increasing the number and competitive fitness of WT ISCs.
Interestingly, Notum plays a similar role in the developing wing disc of Drosophila melanogaster: cells with increased Wnt activity (as a result of APC or Axin mutations) eliminate surrounding cells by triggering apoptosis155. The supercompetition of APC or Axin mutant cells is prevented by genetic mutations inactivating Notum, hence suggesting that the secreted deacylase has an evolutionarily conserved role in Wnt-dependent competition. Finally, the effects of Notum on ISCs are known in the context of the aged intestine. In old Paneth cells, the mammalian target of the rapamycin complex 1 (mTORC1) pathway produces higher levels of Notum, which, in turn, reduces Wnt activity and stem cell numbers, thus limiting the regenerative ability of the aged intestine in response to injury156. This finding provides a possible mechanistic link between age and the increased risk of CRC.
Conclusions
Most studies on cancer biology have focused on the cell-autonomous effects that genetic alterations have on the same cells carrying these mutations. However, it is now evident that cancer cells actively modify their surrounding environment for their own benefit, altering the homeostatic cell competition dynamics to render WT cells as weak competitors. Biased drift, as opposed to neutral competition, accelerates the fixation of certain mutations, from which more aggressive, malignant cancer evolves. Importantly, these changes in cell competition occur through the dysregulation of the same signaling pathways responsible for stem cell maintenance, such as Wnt/β-catenin. Given that Wnt-driven cancers often express high levels of Wnt inhibitors as a negative feedback program, recent discoveries highlighting this expression as a critical mechanism for cancer supercompetition may open up new strategies for pharmacological treatment. Instead of aiming to inhibit Wnt signaling, a difficult task to achieve in APC−/− tumors, it may be more efficient to interfere directly with cancer-derived Wnt antagonists, or vice versa, to increase Wnt activity in WT stem cells, improving their competition capacity. Thus, further studies are needed to address how tumors with different genetic backgrounds modify the niche environment to outcompete the surrounding normal cells.
References
Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15, 19–33 (2014).
Gehart, H. & Clevers, H. Tales from the crypt: New insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 16, 19–34 (2019).
Gordon, J. I. & Hermiston, M. L. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr. Opin. Cell Biol. 6, 795–803 (1994).
Guiu, J. & Jensen, K. B. From definitive endoderm to Gut-a process of growth and maturation. Stem Cells Dev. 24, 1972–1983 (2015).
Cheng, H. & Bjerknes, M. Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat. Rec. 211, 420–426 (1985).
Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284 (2013).
Beumer, J. & Clevers, H. Cell fate specification and differentiation in the adult mammalian intestine. Nat. Rev. Mol. Cell Biol. 22, 39–53 (2021).
Paoli, P., Giannoni, E. & Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta 1833, 3481–3498 (2013).
Guiu, J. et al. Tracing the origin of adult intestinal stem cells. Nature 570, 107–111 (2019).
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am. J. Anat. 141, 461–479 (1974).
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 141, 537–561 (1974).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Kretzschmar, K. & Watt, F. M. Lineage tracing. Cell 148, 33–45 (2012).
van der Flier, L. G., Haegebarth, A., Stange, D. E., van de Wetering, M. & Clevers, H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 137, 15–7 (2009).
van der Flier, L. G. et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136, 903–912 (2009).
Munoz, J. et al. The Lgr5 intestinal stem cell signature: Robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 31, 3079–3091 (2012).
Koo, B. K. et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669 (2012).
Fafilek, B. et al. Troy, a tumor necrosis factor receptor family member, interacts with lgr5 to inhibit wnt signaling in intestinal stem cells. Gastroenterology 144, 381–391 (2013).
Schuijers, J., van der Flier, L. G., van Es, J. & Clevers, H. Robust cre-mediated recombination in small intestinal stem cells utilizing the olfm4 locus. Stem Cell Rep. 3, 234–241 (2014).
Schuijers, J. et al. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 16, 158–170 (2015).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Jaks, V. et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 40, 1291–1299 (2008).
Snippert, H. J. et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 (2010).
Barker, N. et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010).
Plaks, V. et al. Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep. 3, 70–78 (2013).
Ng, A. et al. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat. Cell Biol. 16, 745–757 (2014).
Leung, C. et al. Lgr5 marks adult progenitor cells contributing to skeletal muscle regeneration and sarcoma formation. Cell Rep. 33, 108535 (2020).
Koo, B. K. & Clevers, H. Stem cells marked by the R-spondin receptor LGR5. Gastroenterology 147, 289–302 (2014).
Kim, J., Koo, B. K. & Knoblich, J. A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020).
Fujii, M. & Sato, T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat. Mater. 20, 156–169 (2021).
Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 (2013).
Takahashi, T. & Shiraishi, A. Stem cell signaling pathways in the small intestine. Int. J. Mol. Sci. 21, 2032 (2020).
Potten, C. S., Kovacs, L. & Hamilton, E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 7, 271–283 (1974).
Potten, C. S. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 269, 518–521 (1977).
Potten, C. S., Hume, W. J., Reid, P. & Cairns, J. The segregation of DNA in epithelial stem cells. Cell 15, 899–906 (1978).
Potten, C. S., Owen, G. & Booth, D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 115, 2381–2388 (2002).
Li, L. & Clevers, H. Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545 (2010).
Sangiorgi, E. & Capecchi, M. R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40, 915–920 (2008).
Yan, K. S. et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl Acad. Sci. USA 109, 466–471 (2012).
Montgomery, R. K. et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl Acad. Sci. USA 108, 179–184 (2011).
Takeda, N. et al. Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420–1424 (2011).
Powell, A. E. et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158 (2012).
Tian, H. et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011).
Itzkovitz, S. et al. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat. Cell Biol. 14, 106–114 (2011).
Murata, K. et al. Ascl2-dependent cell dedifferentiation drives regeneration of ablated intestinal stem cells. Cell Stem Cell 26, 377–390 e376 (2020).
Ayyaz, A. et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569, 121–125 (2019).
van Es, J. H. et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 14, 1099–1104 (2012).
Buczacki, S. J. et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69 (2013).
Tetteh, P. W. et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18, 203–213 (2016).
Cheung, P. et al. Regenerative reprogramming of the intestinal stem cell state via Hippo signaling suppresses metastatic colorectal cancer. Cell Stem Cell 27, 590–604 e599 (2020).
Gregorieff, A., Liu, Y., Inanlou, M. R., Khomchuk, Y. & Wrana, J. L. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature 526, 715–718 (2015).
Yui, S. et al. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22, 35–49 e37 (2018).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003).
Takada, R. et al. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev. Cell 11, 791–801 (2006).
Janda, C. Y., Waghray, D., Levin, A. M., Thomas, C. & Garcia, K. C. Structural basis of Wnt recognition by Frizzled. Science 337, 59–64 (2012).
Janda, C. Y. & Garcia, K. C. Wnt acylation and its functional implication in Wnt signalling regulation. Biochem. Soc. Trans. 43, 211–216 (2015).
Nusse, R. & Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Bilic, J. et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316, 1619–1622 (2007).
Colozza, G. & Koo, B. K. Wnt/beta-catenin signaling: Structure, assembly and endocytosis of the signalosome. Dev. Growth Differ. 63, 199–218 (2021).
Taelman, V. F. et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143, 1136–1148 (2010).
Li, V. S. et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell 149, 1245–1256 (2012).
Kim, S. E. et al. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science 340, 867–870 (2013).
Vinyoles, M. et al. Multivesicular GSK3 sequestration upon Wnt signaling is controlled by p120-catenin/cadherin interaction with LRP5/6. Mol. Cell 53, 444–457 (2014).
Colozza, G. et al. Wnt-inducible Lrp6-APEX2 interacting proteins identify ESCRT machinery and Trk-fused gene as components of the Wnt signaling pathway. Sci. Rep. 10, 21555 (2020).
Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13, 767–779 (2012).
Grumolato, L. et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 24, 2517–2530 (2010).
Bottcher, A. et al. Non-canonical Wnt/PCP signalling regulates intestinal stem cell lineage priming towards enteroendocrine and Paneth cell fates. Nat. Cell Biol. 23, 23–31 (2021).
Hu, D. J., Yun, J., Elstrott, J. & Jasper, H. Non-canonical Wnt signaling promotes directed migration of intestinal stem cells to sites of injury. Nat. Commun. 12, 7150 (2021).
van de Moosdijk, A. A. A., van de Grift, Y. B. C., de Man, S. M. A., Zeeman, A. L. & van Amerongen, R. A novel Axin2 knock-in mouse model for visualization and lineage tracing of WNT/CTNNB1 responsive cells. Genesis 58, e23387 (2020).
Korinek, V. et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19, 379–383 (1998).
van de Wetering, M. et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002).
van Es, J. H. et al. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol. Cell Biol. 32, 1918–1927 (2012).
Fevr, T., Robine, S., Louvard, D. & Huelsken, J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell Biol. 27, 7551–7559 (2007).
Glinka, A. et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362 (1998).
Mao, B. et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321–325 (2001).
Pinto, D., Gregorieff, A., Begthel, H. & Clevers, H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17, 1709–1713 (2003).
Kabiri, Z. et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141, 2206–2215 (2014).
Huels, D. J. et al. Wnt ligands influence tumour initiation by controlling the number of intestinal stem cells. Nat. Commun. 9, 1132 (2018).
Farin, H. F., Van, Es,J. H. & Clevers, H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143, 1518–1529 e1517 (2012).
Gregorieff, A. et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129, 626–638 (2005).
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011).
Farin, H. F. et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 530, 340–343 (2016).
Johansson, J. et al. RAL GTPases drive intestinal stem cell function and regeneration through internalization of WNT signalosomes. Cell Stem Cell 24, 592–607 e597 (2019).
Li, Y. et al. Volumetric compression induces intracellular crowding to control intestinal organoid growth via Wnt/beta-catenin signaling. Cell Stem Cell 28, 63–78 e67 (2021).
Cancer Genome Atlas, N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Korinek, V. et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787 (1997).
Morin, P. J. et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275, 1787–1790 (1997).
Liu, W. et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat. Genet. 26, 146–147 (2000).
Bugter, J. M., Fenderico, N. & Maurice, M. M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat. Rev. Cancer 21, 5–21 (2021).
Powell, S. M. et al. APC mutations occur early during colorectal tumorigenesis. Nature 359, 235–237 (1992).
Moser, A. R., Pitot, H. C. & Dove, W. F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324 (1990).
Su, L. K. et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–670 (1992).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47 (2015).
Matano, M. et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 21, 256–262 (2015).
Dow, L. E. et al. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell 161, 1539–1552 (2015).
Ye, P. et al. Tankyrases maintain homeostasis of intestinal epithelium by preventing cell death. PLoS Genet. 14, e1007697 (2018).
Antas, P. et al. SH3BP4 regulates intestinal stem cells and tumorigenesis by modulating beta-catenin nuclear localization. Cell Rep. 26, 2266–2273 e2264 (2019).
Hao, H. X. et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200 (2012).
Jiang, X., Charlat, O., Zamponi, R., Yang, Y. & Cong, F. Dishevelled promotes Wnt receptor degradation through recruitment of ZNRF3/RNF43 E3 ubiquitin ligases. Mol. Cell 58, 522–533 (2015).
Koo, B. K., van Es, J. H., van den Born, M. & Clevers, H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc. Natl Acad. Sci. USA 112, 7548–7550 (2015).
Barker, N., Tan, S. & Clevers, H. Lgr proteins in epithelial stem cell biology. Development 140, 2484–2494 (2013).
Glinka, A. et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055–1061 (2011).
Kazanskaya, O. et al. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev. Cell 7, 525–534 (2004).
Carmon, K. S., Gong, X., Lin, Q., Thomas, A. & Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl Acad. Sci. USA 108, 11452–11457 (2011).
Park, S. et al. Differential activities and mechanisms of the four R-spondins in potentiating Wnt/beta-catenin signaling. J. Biol. Chem. 293, 9759–9769 (2018).
de Lau, W. et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 (2011).
Hao, H. X., Jiang, X. & Cong, F. Control of Wnt receptor turnover by R-spondin-ZNRF3/RNF43 signaling module and its dysregulation in cancer. Cancers 8, 54 (2016).
Kim, K. A. et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309, 1256–1259 (2005).
Yan, K. S. et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature 545, 238–242 (2017).
Hilkens, J. et al. RSPO3 expands intestinal stem cell and niche compartments and drives tumorigenesis. Gut 66, 1095–1105 (2017).
Seshagiri, S. et al. Recurrent R-spondin fusions in colon cancer. Nature 488, 660–664 (2012).
Neumeyer, V. et al. Loss of endogenous RNF43 function enhances proliferation and tumour growth of intestinal and gastric cells. Carcinogenesis 40, 551–559 (2019).
Eto, T. et al. Impact of loss-of-function mutations at the RNF43 locus on colorectal cancer development and progression. J. Pathol. 245, 445–455 (2018).
Min, B. H. et al. Dysregulated Wnt signalling and recurrent mutations of the tumour suppressor RNF43 in early gastric carcinogenesis. J. Pathol. 240, 304–314 (2016).
Bond, C. E. et al. RNF43 and ZNRF3 are commonly altered in serrated pathway colorectal tumorigenesis. Oncotarget 7, 70589–70600 (2016).
Giannakis, M. et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 46, 1264–1266 (2014).
Wang, K. et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 46, 573–582 (2014).
Jiang, X. et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc. Natl Acad. Sci. USA 110, 12649–12654 (2013).
Steinhart, Z. et al. Genome-wide CRISPR screens reveal a Wnt-FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors. Nat. Med. 23, 60–68 (2017).
Tsukiyama, T., Koo, B. K. & Hatakeyama, S. Post-translational Wnt receptor regulation: Is the fog slowly clearing?: The molecular mechanism of RNF43/ZNRF3 ubiquitin ligases is not yet fully elucidated and still controversial. Bioessays 43, e2000297 (2021).
Tsukiyama, T. et al. A phospho-switch controls RNF43-mediated degradation of Wnt receptors to suppress tumorigenesis. Nat. Commun. 11, 4586 (2020).
Spit, M. et al. RNF43 truncations trap CK1 to drive niche-independent self-renewal in cancer. EMBO J. 39, e103932 (2020).
Chang, L. S., Kim, M., Glinka, A., Reinhard, C. & Niehrs, C. The tumor suppressor PTPRK promotes ZNRF3 internalization and is required for Wnt inhibition in the Spemann organizer. Elife 9, e51248 (2020).
Giebel, N. et al. USP42 protects ZNRF3/RNF43 from R-spondin-dependent clearance and inhibits Wnt signalling. EMBO Rep. 22, e51415 (2021).
Colozza, G. & Koo, B. K. Ub and Dub of RNF43/ZNRF3 in the WNT signalling pathway. EMBO Rep. 22, e52970 (2021).
Fuller, M. T. & Spradling, A. C. Male and female Drosophila germline stem cells: Two versions of immortality. Science 316, 402–404 (2007).
Betschinger, J. & Knoblich, J. A. Dare to be different: Asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674–R685 (2004).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Lopez-Garcia, C., Klein, A. M., Simons, B. D. & Winton, D. J. Intestinal stem cell replacement follows a pattern of neutral drift. Science 330, 822–825 (2010).
Ritsma, L. et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507, 362–365 (2014).
Winton, D. J. & Ponder, B. A. Stem-cell organization in mouse small intestine. Proc. Biol. Sci. 241, 13–18 (1990).
Griffiths, D. F., Davies, S. J., Williams, D., Williams, G. T. & Williams, E. D. Demonstration of somatic mutation and colonic crypt clonality by X-linked enzyme histochemistry. Nature 333, 461–463 (1988).
Degirmenci, B., Valenta, T., Dimitrieva, S., Hausmann, G. & Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 558, 449–453 (2018).
Shoshkes-Carmel, M. et al. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 557, 242–246 (2018).
Greicius, G. et al. PDGFRalpha(+) pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc. Natl Acad. Sci. USA 115, E3173–E3181 (2018).
Bahar Halpern, K. et al. Lgr5+ telocytes are a signaling source at the intestinal villus tip. Nat. Commun. 11, 1936 (2020).
Kim, J. E. et al. Single cell and genetic analyses reveal conserved populations and signaling mechanisms of gastrointestinal stromal niches. Nat. Commun. 11, 334 (2020).
McCarthy, N. et al. Distinct mesenchymal cell populations generate the essential intestinal BMP signaling gradient. Cell Stem Cell 26, 391–402 e395 (2020).
Wu, N. et al. MAP3K2-regulated intestinal stromal cells define a distinct stem cell niche. Nature 592, 606–610 (2021).
Leushacke, M., Ng, A., Galle, J., Loeffler, M. & Barker, N. Lgr5(+) gastric stem cells divide symmetrically to effect epithelial homeostasis in the pylorus. Cell Rep. 5, 349–356 (2013).
Han, S. et al. Defining the identity and dynamics of adult gastric isthmus stem cells. Cell Stem Cell 25, 342–356 e347 (2019).
Liu, N. et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 568, 344–350 (2019).
Merino, M. M. et al. Elimination of unfit cells maintains tissue health and prolongs lifespan. Cell 160, 461–476 (2015).
Tanimura, N. & Fujita, Y. Epithelial defense against cancer (EDAC). Semin. Cancer Biol. 63, 44–48 (2020).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Vermeulen, L. et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science 342, 995–998 (2013).
Snippert, H. J., Schepers, A. G., van Es, J. H., Simons, B. D. & Clevers, H. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep. 15, 62–69 (2014).
Yum, M. K. et al. Tracing oncogene-driven remodelling of the intestinal stem cell niche. Nature 594, 442–447 (2021).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
van Neerven, S. M. et al. Apc-mutant cells act as supercompetitors in intestinal tumour initiation. Nature 594, 436–441 (2021).
Flanagan, D. J. et al. NOTUM from Apc-mutant cells biases clonal competition to initiate cancer. Nature 594, 430–435 (2021).
Kakugawa, S. et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature 519, 187–192 (2015).
Zhang, X. et al. Notum is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev. Cell 32, 719–730 (2015).
Vincent, J. P., Kolahgar, G., Gagliardi, M. & Piddini, E. Steep differences in wingless signaling trigger Myc-independent competitive cell interactions. Dev. Cell 21, 366–374 (2011).
Pentinmikko, N. et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 571, 398–402 (2019).
Acknowledgements
We thank the members of the Koo laboratory for critically reading the manuscript. We thank SunghWan Bae of BSTAR ARTWORK for assistance in preparing the figures. G.C. is supported by the Austrian Science Fund (FWF), Lise Meitner Program M 2976. This work is dedicated to the memory of Maurizio Colozza (1950–2021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Colozza, G., Park, SY. & Koo, BK. Clone wars: From molecules to cell competition in intestinal stem cell homeostasis and disease. Exp Mol Med 54, 1367–1378 (2022). https://doi.org/10.1038/s12276-022-00854-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-022-00854-5