Abstract
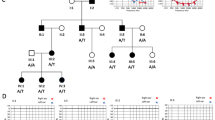
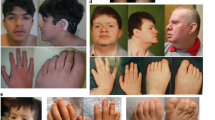
Heterozygous pathogenic variants in SLC12A2 are reported in patients with nonsyndromic hearing loss. Recently, homozygous loss-of-function variants have been reported in two patients with syndromic intellectual disability, with or without hearing loss. However, the clinical and molecular spectrum of SLC12A2 disease has yet to be characterized and confirmed. Using whole-exome sequencing, we detected a homozygous splicing variant in four patients from two independent families with severe developmental delay, microcephaly, respiratory abnormalities, and subtle dysmorphic features, with or without congenital hearing loss. We also reviewed the reported cases with pathogenic variants associated with autosomal dominant and recessive forms of the SLC12A2 disease. About 50% of the cases have syndromic and nonsyndromic congenital hearing loss. All patients harboring the recessive forms of the disease presented with severe global developmental delay. Interestingly, all reported variants are located in the c-terminal domain, suggesting a critical role of this domain for the proper function of the encoded co-transporter protein. In conclusion, our study provides an additional confirmation of the autosomal recessive SLC12A2 disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Delpire E, Gagnon KB. Na(+) -K(+) -2Cl(-) Cotransporter (NKCC) physiological function in nonpolarized cells and transporting epithelia. Compr Physiol. 2018;8:871–901.
Nin F, Yoshida T, Murakami S, Ogata G, Uetsuka S, Choi S. et al. Computer modeling defines the system driving a constant current crucial for homeostasis in the mammalian cochlea by integrating unique ion transports. NPJ Syst Biol Appl. 2017;3:24
Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11–21.
Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA. et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–13.
Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–8.
De Koninck Y. Altered chloride homeostasis in neurological disorders: a new target. Curr Opin Pharm. 2007;7:93–99.
Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE. et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–95.
Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP. et al. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pr Neurol. 2008;4:490–503.
Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J. et al. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell. 2012;148:1051–64.
Payne JA. Molecular operation of the cation chloride cotransporters: ion binding and inhibitor interaction. Curr Top Membr. 2012;70:215–37.
Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet. 1999;22:192–5.
Dixon MJ, Gazzard J, Chaudhry SS, Sampson N, Schulte BA, Steel KP. Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum Mol Genet. 1999;8:1579–84.
Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A. et al. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–55.
Young SZ, Taylor MM, Wu S, Ikeda-Matsuo Y, Kubera C, Bordey A. NKCC1 knockdown decreases neuron production through GABA(A)-regulated neural progenitor proliferation and delays dendrite development. J Neurosci. 2012;32:13630–8.
Marchese M, Valvo G, Moro F, Sicca F, Santorelli FM. Targeted gene resequencing (astrochip) to explore the tripartite synapse in autism-epilepsy phenotype with macrocephaly. Neuromolecular Med. 2016;18:69–80.
Merner ND, Mercado A, Khanna AR, Hodgkinson A, Bruat V, Awadalla P. et al. Gain-of-function missense variant in SLC12A2, encoding the bumetanide-sensitive NKCC1 cotransporter, identified in human schizophrenia. J Psychiatr Res. 2016;77:22–6.
Delpire E, Wolfe L, Flores B, Koumangoye R, Schornak CC, Omer S. et al. A patient with multisystem dysfunction carries a truncation mutation in human SLC12A2, the gene encoding the Na-K-2Cl cotransporter, NKCC1. Cold Spring Harb Mol Case Stud. 2016;2:a001289
Anazi S, Maddirevula S, Salpietro V, Asi YT, Alsahli S, Alhashem A. et al. Expanding the genetic heterogeneity of intellectual disability. Hum Genet. 2017;136:1419–29.
Macnamara EF, Koehler AE, D’Souza P, Estwick T, Lee P, Vezina G. et al. Kilquist syndrome: a novel syndromic hearing loss disorder caused by homozygous deletion of SLC12A2. Hum Mutat. 2019;40:532–8.
Alhebbi H, Peer-Zada AA, Al-Hussaini AA, Algubaisi S, Albassami A, AlMasri N, et al. New paradigms of USP53 disease: normal GGT cholestasis, BRIC, cholangiopathy, and responsiveness to rifampicin. J Hum Genet. 2020;66:151–9.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Mutai H, Wasano K, Momozawa Y, Kamatani Y, Miya F, Masuda S. et al. Variants encoding a restricted carboxy-terminal domain of SLC12A2 cause hereditary hearing loss in humans. PLoS Genet. 2020;16:e1008643
Clayton GH, Owens GC, Wolff JS, Smith RL. Ontogeny of cation-Cl- cotransporter expression in rat neocortex. Brain Res Dev Brain Res. 1998;109:281–92.
Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–24.
Pfeffer CK, Stein V, Keating DJ, Maier H, Rinke I, Rudhard Y. et al. NKCC1-dependent GABAergic excitation drives synaptic network maturation during early hippocampal development. J Neurosci. 2009;29:3419–30.
Tao R, Li C, Newburn EN, Ye T, Lipska BK, Herman MM. et al. Transcript-specific associations of SLC12A5 (KCC2) in human prefrontal cortex with development, schizophrenia, and affective disorders. J Neurosci. 2012;32:5216–22.
Callicott JH, Feighery EL, Mattay VS, White MG, Chen Q, Baranger DA. et al. DISC1 and SLC12A2 interaction affects human hippocampal function and connectivity. J Clin Invest. 2013;123:2961–4.
Gagnon KB, Delpire E. Physiology of SLC12 transporters: lessons from inherited human genetic mutations and genetically engineered mouse knockouts. Am J Physiol Cell Physiol. 2013;304:C693–714.
Sun Q, Tian E, Turner RJ, Ten, Hagen KG. Developmental and functional studies of the SLC12 gene family members from Drosophila melanogaster. Am J Physiol Cell Physiol. 2010;298:C26–37.
Acknowledgements
We thank the study families for their participation. The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number 821.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bilal Shamsi, M., Saleh, M., Almuntashri, M. et al. Clinical characterization and further confirmation of the autosomal recessive SLC12A2 disease. J Hum Genet 66, 689–695 (2021). https://doi.org/10.1038/s10038-021-00904-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-021-00904-2