Abstract
BACKGROUND
The aims of this study were to (i) compare the concentrations of two neural injury markers, S100B protein and neuron-specific enolase (NSE), in intrauterine growth-restricted (IUGR) fetuses and in fetuses with appropriate growth-for-gestational-age (AGA), and (ii) investigate potential relationships between concentrations of these markers, Doppler abnormalities, and adverse perinatal or neonatal outcomes.
METHODS
This was a case-controlled, cooperative, prospective study among Spanish Maternal and Child Health Network (Retic SAMID) hospitals. At inclusion, biometry for estimated fetal weight and feto-placental Doppler were measured. At the time of delivery, maternal venous blood and fetal umbilical arterial blood samples were collected. S100B and NSE concentrations were determined from these samples.
RESULTS
In total, 254 pregnancies were included. Among these, 147 were classified as IUGR and 107 as AGA. There were no differences between the groups in S100B concentrations. However, levels of NSE in maternal and umbilical cord serum differed significantly between these groups (2.31 in AGA vs. 2.51 in IUGR in (P<0.05); and 2.89 in AGA vs. 3.25 in IUGR (P<0.05), respectively). No differences were observed in these neurological markers when stratified by perinatal or neonatal complications.
CONCLUSION
Although some variations exist in these neurological markers, they did not correlate with perinatal or neonatal complications.
Similar content being viewed by others
Main
Intrauterine growth restriction (IUGR) refers to the failure of a fetus to achieve its endowed growth potential, and, in most cases, it is secondary to placental insufficiency. In general, IUGR is associated with Doppler signs suggesting hemodynamic redistribution, as a reflection of fetal adaptation to undernutrition/hypoxia, histological and biochemical signs of placental disease, and a higher risk of preeclampsia (1).
Fetuses with IUGR have poorer perinatal outcomes and a higher risk for fetal in utero deterioration and stillbirth compared with fetuses without IUGR (1). In addition, IUGR has long-term metabolic, neurologic, and cardiovascular consequences (2, 3, 4, 5, 6). Among these, neurodevelopment has received considerable attention because of its impact on the quality of life and because the IUGR morbidity is strongly dependent on fetal central nervous system injury (7, 8). Therefore, the availability of reliable antenatal or neonatal prognostic markers of such complications might improve parental counseling in cases of IUGR.
Neural injury markers are proteins associated with neural tissue. Their release in the plasma increases when neural injury, such as cerebral hypoxia, occurs. Thus, measuring brain-related products associated with neural injury can help predict which fetuses or neonates are at risk for pathological effects to the nervous system (9).
One of these neural injury markers is S100B, which was first identified in 1965 (ref. 9). S100B is an acidic calcium-binding protein found in glial cells, astrocytes, Schwann cells, and neurons. Currently, results described in the literature about S100B are somewhat contradictory. Elevated S100B in biological fluids, such as cerebrospinal fluid, cord blood, peripheral blood, and urine, has been identified as a marker of brain damage in some studies (10). Levels of S100B are also higher in neonates with Doppler abnormalities, which suggest that S100B could be a good marker for neural injury that is secondary to chronic cerebral hypoxia (11). Moreover, high maternal S100B levels could be associated with a higher risk of neonatal intraventricular hemorrhage in intrauterine growth-restricted fetuses (12) and a higher risk of neurological disabilities in preterm babies (13).
However, many other studies have demonstrated contradictory results. Boutsikou et al. (14) did not find any significant differences in maternal serum or cord blood levels of S100B between IUGR and appropriate growth-for-gestational-age (AGA) cases; and Kiseli et al. (15) also showed that S100B was not increased in umbilical cord blood of IUGR fetuses.
Another neural injury marker is neuron-specific enolase (NSE). NSE is a dimeric isoenzyme of the glycolytic enzyme enolase, which is primarily located in the cytosol of neurons and neuroendocrine cells. Although NSE has been studied mainly in cerebrospinal fluid, serum levels in adult patients with cerebral infarction correlate with acute neurological symptoms, lesion volume, and long-term evolution. In newborns, NSE has been shown to predict the neurological development of children who suffered asphyxia in the perinatal period, and it can be used to estimate the severity of brain damage in congenital cytomegalovirus infection (16). In addition, high fetal amniotic NSE levels are associated with neonatal intraventricular hemorrhage (17, 18) and with neonatal mortality, necrotizing enterocolitis (NEC), and the need for intubation (9). Despite NSE being a good neural injury marker, not many studies have investigated NSE in IUGR.
The aim of this study was to show the distribution of two neural injury markers, S100B and NSE, in maternal and fetal umbilical serum of IUGR fetuses and in fetuses AGA and to compare them. This study also investigated the correlation between concentrations of these markers, Doppler abnormalities, and adverse perinatal or neonatal outcomes.
METHODS
This is a case–control prospective study performed cooperatively in Spanish Maternal and Child Health Network (RETIC SAMID) hospitals including Hospital Sant Joan de Deú in Barcelona, Hospital Vall Hebrón in Barcelona, and Hospital Cruces in Bilbao.
Subjects
Consecutive cases of IUGR singleton pregnancies were recruited over a 12-month period from women attending the outpatient clinic in the obstetrics department of the hospitals listed above. These cases were matched by gestational age (±7 days) with AGA (fetal weight 10–90th centile (19)) pregnancies.
IUGR was confirmed after birth in all newborns fulfilling the following criteria:
-
1)
Birth weight <3rd centile with normal Doppler (20),
-
2)
Birth weight <10th centile with abnormal uterine arterial Doppler study (pulsatility index (PI) >95th centile) (21), or
-
3)
Birth weight <10th centile with abnormal cerebroplacental ratio (CPR; CPR <5th centile) (22).
Exclusion criteria were the following: use of illicit drugs, maternal endocrine pathological conditions that might interfere with fetal growth, fetal infections, pregnancies with multiple fetuses, pregnancies with fetal malformations or genetic anomalies, failure of the mother to follow the clinical protocols, and delivery in a hospital other than the ones listed previously.
All cases were dated by measurement of the crown–rump length during the first trimester (23).
The study protocol was approved by the Institutional Review Board of Sant Joan de Déu University Hospital and by local Ethics Committees from Hospital Vall Hebrón and Hospital Cruces. Written informed consent was obtained from each participant.
Doppler Measurements
In all cases, fetal biometry and prenatal Doppler ultrasound examinations were performed by experienced operators using a General Electric Voluson E8 (GE Medical Systems, Zipf, Austria) ultrasound machine equipped with a 6–2 MHz, linear curved-array transducer. The estimated fetal weight was calculated from the biparietal diameter, head and abdominal circumferences, and femur length using the Hadlock formula (24). The umbilical artery (UA) PI was calculated from a free-floating portion of the umbilical cord. To minimize variability, the middle cerebral artery (MCA) PI was measured in a transverse view of the fetal head, at the level of its origin from the circle of Willis (25). The CPR was calculated as the ratio of the MCA PI divided by the UA PI (26). For uterine artery (UtA) assessment, the ultrasound probe was placed on the lower quadrant of the abdomen, angled medially, and color Doppler imaging was used to identify the UtA at the apparent crossover with the external iliac artery. Measurements were taken ~1 cm distal to the crossover point. Doppler readings were recorded during the absence of fetal movements and maternal breathing was voluntarily suspended. All pulsed Doppler parameters were recorded automatically from at least three consecutive waveforms, with the angle of insonation as close to 0 as possible and always below 30°. A high-pass wall filter of 70 Hz was used to record low flow velocities and to avoid artifacts. The last Doppler evaluation within 1 week of delivery was included in the analysis.
S100 and NSE Measurements
At the time of delivery, maternal venous blood and fetal umbilical arterial blood samples were collected. Blood samples were centrifuged at 3,000 rpm for 10 min, and supernatants were collected and stored at −80 °C for biochemical analysis. Samples from Hospital Vall Hebrón and Hospital Cruces were adequately transported to Hospital Sant Joan de Deú, and the biochemical analysis from all the samples was performed in this center.
Serum S100B protein was measured by LIAISON S100 (DiasSorin S.p.A, Saluggia, Italy), which is a quantitative automated sandwich chemiluminescent immunoassay for the in vitro determination of protein S100B in human serum. The analytical range of the test is 0.02–30 μg/l; and it requires two steps, first using directly coated magnetic particles (solid phase) and then by an isoluminol derivative (conjugate). The total time for incubation was 20 and 10 min, respectively, for each step.
Serum NSE was measured by LIAISON NSE (DiasSorin S.p.A), which is an in vitro assay for the quantitative determination of NSE in human serum. The analytical range of the test is 0.04–200 μg/l. The assay was conducted in two steps—first using directly coated magnetic particles (solid phase) and then an isoluminol derivative (conjugate). The total time for incubation was 10 min.
Neonatal Assessment
At the time of birth, 1- and 5-min Apgar scores and umbilical cord pH were recorded. During newborn hospitalization, the following items were recorded: somatometric measures, neonatal mortality (early/late), respiratory distress syndrome (need for oxygen after 4 h of life, continuous positive airway pressure, mechanical ventilation, and/or surfactant administration), altered neurological examination at discharge, gastrointestinal intolerance lasting more than 2 days, NEC or isolated intestinal perforation, sepsis (early/late), bronchopulmonary dysplasia (oxygen requirement at 36 weeks), intraventricular hemorrhage grade III or IV, periventricular leukomalacia, retinopathy of prematurity, or patent ductus arteriosus that required pharmacological or surgical closure.
Perinatal complications included the following: cesarean section during labor, acidosis or 5 min Apgar ⩽7, and neonatal complications such as respiratory distress syndrome, intraventricular hemorrhage, periventricular leukomalacia, NEC, hyaline membrane disease, sepsis, bronchopulmonary dysplasia, retinopathy, meningitis, abnormal neurological examination, or digestive intolerance.
Statistical Analysis
Quantitative variables were log-transformed to achieve a normal distribution, which was evaluated by the Shapiro–Wilk test. Quantitative and qualitative variables were compared between groups by one-way analysis of variance (assuming equal or unequal variances by Levene’s test) and χ2-tests (or Fisher’s exact test when cell frequencies were <5), respectively. Correlations were evaluated bivariately by Pearson’s test and by linear regression for adjustment by gestational age at evaluation. A P value of <0.05 was considered statistically significant. Statistical analyses were conducted using Stata software v13.0 (Stata Corp., College Station, TX), and graphs were constructed with R v3.1.2 (The R Foundation for Statistical Computing; package “beewarm 0.1.6”) (27).
RESULTS
A total of 147 pregnancies were identified as IUGR and 107 as AGA. Tables 1 and 2 show the baseline characteristics of the study population and the perinatal and neonatal outcomes by study group. As expected, IUGR cases had a higher incidence of preeclampsia than AGA cases had. Noticeably, IUGR cases were delivered earlier, resulting in babies with a lower birth weight centile. In addition, IUGR babies had a higher incidence of perinatal and neonatal complications and longer stay in the neonatal intensive care unit.
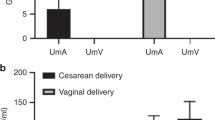
On comparing the concentrations of S100B and NSE in maternal serum and umbilical cord blood between the IUGR and AGA groups, we found no significant differences in the concentrations of S100B (−1.53 in AGA vs. −1.4 in IUGR in maternal serum (log-transformed) (P>0.05) and 0.03 in AGA vs. 0.22 in IUGR in umbilical cord blood (log-transformed) (P>0.05)). However, levels of NSE in maternal serum and umbilical cord blood significantly differed between the groups, with higher values in IUGR cases (2.31 in AGA vs. 2.51 in IUGR in maternal serum (log-transformed) (P<0.05) and 2.89 in AGA vs. 3.25 in IUGR in umbilical cord blood (log-transformed) (P<0.05)). Figures 1 and 2 graphically show the distribution of these markers.
While investigating the correlation between the concentrations of S100B in either maternal serum or cord blood and Doppler parameters, after adjusting by gestational age at birth, we found no correlations. However, we found that maternal circulating levels of enolase significantly correlated with the CPR and the UA PI (P<0.05); and umbilical cord levels of enolase significantly correlated with the CPR, UA PI, and UtA PI (P<0.05).
Neither marker in maternal serum nor cord blood had significantly different concentrations (P>0.05) when a perinatal (cesarean section during labor, acidosis, or 5-min Apgar ⩽7) or neonatal (respiratory distress syndrome, intraventricular hemorrhage, periventricular leukomalacia, NEC, hyaline membrane disease, sepsis, bronchopulmonary dysplasia, retinopathy, meningitis, abnormal neurologic examination, or digestive intolerance) complication occurred. Regarding neurologic morbidity, there are no cases of intraventricular hemorrhage; however, there are 11 cases of periventricular leukomalacia (two AGA and nine IUGR). There were also no differences in S100B or NSE concentrations in these cases.
DISCUSSION
Our study shows that, although some changes may exist between neurological markers in IUGR fetuses, no differences were observed between neurological markers when stratified by perinatal or neonatal complications.
Contrary to our results, many studies have found correlations between S100B concentrations and neural injury due to chronic cerebral hypoxia in IUGR. Gazzolo et al. (11) demonstrated higher levels of S100B in umbilical blood of newborns with IUGR. Moreover, increased concentrations of S100B after cell injury in the nervous system have supported the use of S100B as a marker of hypoxia-induced brain damage (10, 28), and clinical studies reported that S100B has a sensitivity of 95% and a specificity of 99.1% for predicting an adverse neurological outcome in IUGR fetuses (10), and higher concentrations of S100B have been found in maternal blood of IUGR fetuses with intraventricular hemorrhage (12). In addition, redistribution of the blood flow, as detected by increased resistance in the umbilical artery and decreased resistance in the MCA Doppler flow, increases levels of S100B in growth-retarded fetuses (11). And in growth-retarded newborns, increased urinary levels of S100B protein suggest the presence of brain damage secondary to intrauterine hypoxia (10) along with the abnormalities in MCA and umbilical artery Doppler (29, 30, 31).
Our study, however, showed no significant difference between the IUGR and AGA groups in regard to S100B concentrations in maternal serum or umbilical cord blood; and we found no correlations between the S100B concentrations in either maternal serum or umbilical blood and Doppler parameters. Consistent with our results, Boutsikou et al. (14) and Kiseli et al. (15) found no significant differences in S100B levels between IUGR and AGA cases.
One possible explanation for the non-consistent results reported in the literature is that different severity stages of hypoxia could be present. Brain-sparing phenomena could ensure adequate nutrient supply to vital organs and maintain glial cells, even in the presence of malnutrition. Therefore, these changes could only be observed in severely deteriorated cases.
In our study, IUGR infants showed better outcomes (good Apgar scores, good arterial pH at birth, and no neurologic sequelae in the short term) compared with those of Gazzolo et al. (11, 12, 29), Florio et al. (10), and Constantine et al. (31). Moreover, our cases had no significant differences in Apgar scores or umbilical artery pH at birth compared with controls; in addition, we had no cases of perinatal mortality or intraventricular hemorrhage grade III or IV, pointing out that our cohort has a relatively early stage of deterioration. Regarding neurologic morbidity, we had 11 cases of periventricular leukomalacia and S100B or NSE concentrations were also the same in these cases. On the other hand, the IUGR groups reported by Gazzolo et al. (11, 12, 29), Florio et al. (10), and Constantine et al. (31) had significant differences in Apgar scores at 1 and 5 min and in perinatal mortality and intraventricular hemorrhage grade III or IV from their respective control groups.
Regarding NSE, few studies have investigated NSE in IUGR. High fetal amniotic NSE levels are associated with neonatal intraventricular hemorrhage (17, 18) and with neonatal mortality, NEC, and need for intubation (9).
In our study, NSE levels in maternal serum and umbilical cord blood significantly differed between groups and correlated with Doppler parameters (CPR, UA PI, and UtA PI). However, no correlation was found between NSE levels and perinatal or neonatal complications. Our results differ from those reported by Velipasaoglu et al. (9), as they found that NSE levels were significantly associated with neonatal mortality, NEC, and need for intubation. Once again, this discrepancy might be explained by the difference in IUGR-group characteristics: that study included an IUGR group with a higher incidence of neonatal complications (28.1% neonatal death, 18.8% NEC, 34.4% need for intubation, and 9.4% intraventricular hemorrhage) compared with our IUGR group. From our results, NSE appeared to be more sensitive than S100B as a marker in IUGR cases and especially for those fetuses more affected, as reflected by NSE’s correlation with the CPR, UA PI, and UtA PI.
In conclusion, our study shows that, although some changes may exist in neurological markers in IUGR fetuses, especially in those with more severe Doppler hypoxia stages, no significant correlations with neonatal complications were observed. Follow-up studies could examine whether these markers would predict long-term neurological impairment.
References
Figueras F, Gratacós E . Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther 2014;36:86–98.
Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A . Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol 2000;182:198–206.
Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME . Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989;298:564–7.
Osmond C, Barker DJ . Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect 2000;108:545–53.
Kanaka C, Mastorakos G, Chrousos GP . Endocrine-related causes and consequences of intrauterine growth retardation. Ann NY Acad Sci 2003;997:150–7.
Barker DJ . Adult consequences of fetal growth restriction. Clin Obstet Gynecol 2006;49:270–83.
Hagberg H, Mallard C . Antenatal brain injury: aetiology and possibilities of prevention. Semin Neonatol 2000;5:41–51.
Baschat A, Viscardi M, Hussey-Gardner C, Hashmi N, Harman C . Infant neurodevelopment following fetal growth restriction: relationship with antepartum surveillance parameters. Ultrasound Obstet Gynecol 2009;33:44–50.
Velipasaoglu M, Yurdakok M, Ozyuncu O, Portakal O, Deren O . Neural injury markers to predict neonatal complications in intrauterine growth restriction. J Obstet Gynecol 2015;35:555–60.
Florio P, Marinoni E, Di Iorio R et al, Urinary S100B protein concentracions are increased in intrauterine growth-retarded newborns. Pediatrics 2006;118:747–54.
Gazzolo D, Visser GHS, Lituania M et al, S100B protein cord blood levels and development of fetal behavioral states: a study in normal and small-for-dates fetuses. J Maternal Fetal Neonatal Med 2002;11:378–84.
Gazzolo D, Marinoni E, Di Iorio R et al, High maternal blood S100B concentrations in pregnancies complicated by intrauterine growth restriction and intraventricular hemorrhage. Clin Chem 2006;52:819–826.
Serpero L, Pluchinotta F, Gazzolo D . The clinical and diagnostic utility of S100B in preterm newborns. Clin Chim Acta 2014;444:193–8.
Boutsikou T, Mastorakos G, Kyriakakou M et al, Circulating levels of inflammatory markers in intrauterine growth restriction. Mediators Inflamm 2010;2010:790605.
Kiseli M, Caglar GS, Gursoy AY, Ozdemir H, Seker ET, Demirtas S . Maternal and fetal blood levels of S100 and ischaemia modified albumin interm intrauterine growth restricted fetuses with abdnormal umbilical artery Doppler values. J Obstet Gynecol 2015;35:368–71.
Giuseppe D, Sergio C, Pasqua B et al, Perinatal asphyxia in preterm neonates leads to serum changes in protein S-100 and neuron specific enolase. Curr Neurovasc Res 2009;6:110–6.
Elimian A, Figueroa R, Verma U, Visintainer P, Sehgal PB, Tejani N . Amniotic fluid neuron-specific enolase: a role in predicting neonatal neurologic injury. Obstet Gynecol 1998;92:546–550.
Wijnberger L, Nikkels P, van Dongen A et al, Expression in the placenta of neural markers for perinatal brain damage. Pediatr Res 2002;51:492–6.
Figueras F, Meler E, Iraola A, Eixarch E, Coll O . Customized birthweight standards for a Spanish population. Eur J Obstet Gynecol Reprod Biol 2008;136:20–4.
Figueras F, Gratacós E . Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther 2014;36:86–98.
Arduini D, Rizzo G . Normal values of pulsatility index from fetal vessels: a cross-sectional study on 1556 healthy fetuses. J Perinat Med 1990;18:165–72.
Baschat AA, Gembruch U . The cerebroplacental Doppler ratio revisited. Ultrasound Obstet Gynecol 2003;21:124–7.
Robinson HP, Fleming JE . A critical evaluation of sonar “crown-rump length” measurements. Br J Obstet Gynaecol. 1975;82:702–10.
Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK . Estimation of fetal weight with the use of head, body, and femur measurements – a prospective study. Am J Obstet Gynecol 1985;151:333–7.
Mari G, Moise KJ, Deter RL, Kirshon B, Carpenter RJ, Huhta J . Doppler assessment of the pulsatility index in the cerebral circulation of the human fetus. Am J Obstet Gynecol 1989;160:698–703.
Gramellini D, Folli MC, Raboni S, Vadora E, Merialdi A . Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol 1992;74:416–20.
Qian J, Zhou D, Wang YW . Umbilical artery blood S100 beta protein: a tool for the early identification of neonatal hypoxic-ischemic encephalopathy. Eur J Pediatr 2009;168:71–77.
Jang DG, Jo YS, Lee SJ, Kim N, Lee GS . Perinatal outcomes and maternal clinical characteristics in IUGR with absent or reversed end-diastolic flow velocity in the umbilical artery. Arch Gynecol Obstet 2011;284:73–8.
Gazzolo D, Marinoni E, Di Iorio R et al, Measurement of urinary S100B protein concentrations for the early identification of brain damage in asphyxiated full-term infants. Arch Pediatr Adolesc Med 2003;157:1163–8.
Gazzolo D, Marinoni E, Di Iorio R et al, Urinary S100B protein measurements: a tool for the early identification of hypoxic-ischemic encephalopathy in asphyxiated full-term infants. Crit Care Med 2004;32:131–6.
Costantine MM, Weiner SJ, Rouse DJ et al, Umbilical cord blood biomarkers of neurologic injury and the risk of cerebral palsy or infant death. Int J Dev Neurosci 2011;29:917–22.
Acknowledgements
We thank the Gynecology and Obstetrics Service at the Sant Joan de Déu University Hospital in Barcelona and the RETICS funded by the PN I+D+I 2008-2011 (Spain), ISCIII- Sub-Directorate General for Research Assessment and Promotion, and the European Regional Development Fund (ERDF), ref. RD12/0026, and the Fund for Health Research of the Spanish Social Security Service for financing part of this project (Exp. PI11/02613).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of financial support
This project was supported in part by the European Regional Development Fund (ERDF), ref. RD12/0026, and the Fund for Health Research of the Spanish Social Security Service (Exp. PI11/02613). Each of the authors has reviewed the final version of the manuscript, and all have given their consent for this submission.
Rights and permissions
About this article
Cite this article
Mazarico, E., Llurba, E., Cumplido, R. et al. Neural injury markers in intrauterine growth restriction and their relation to perinatal outcomes. Pediatr Res 82, 452–457 (2017). https://doi.org/10.1038/pr.2017.108
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.108