Abstract
Introduction:
To our knowledge, elucidating the immune pathogenesis of disease, especially characteristic T-cell and natural killer (NK) cell expansions, has not been performed before now. We investigated the role of T lymphocytes and NK lymphocytes in the destruction of extrahepatic bile ducts of patients with biliary atresia.
Methods:
Lymphocytes from the liver and extrahepatic bile duct remnants of patients with biliary atresia were characterized by immunofluorescence staining, fluorescence-activated cell sorter analysis, and real-time reverse-transcriptase PCR. Cholangiocyte lysis assays were performed to confirm cytotoxicity of activated hepatic NK lymphocytes or CD8+ cells.
Results:
The inflammatory milieu from portal tracts and/or biliary remnants consisted of greater numbers of Kupffer cells, T lymphocytes, and NK lymphocytes in the patients with biliary atresia as compared with the cholestatic and noncholestatic controls. In patients with biliary atresia, expression of NK or CD8+ costimulatory molecules was upregulated as compared with controls. Hepatic NK lymphocytes or CD8+ cells from patients with biliary atresia were demonstrated to be cytotoxic to the duct epithelium.
Discussion:
Specific immune responses from NK and CD8+ cells were involved in the injury to the duct epithelium and play a significant role in the phenotype of experimental biliary atresia.
Similar content being viewed by others
Main
Biliary atresia (BA) is the most common cause of chronic liver disease in infants. The most commonly observed symptom is pathologic jaundice during the neonatal period. Early diagnosis and prompt surgical construction of a portoenterostomy to improve bile drainage are critical in the treatment of BA. Although the Kasai procedure has been performed since the 1950s, this method is unable to stop progression to end-stage cirrhosis in infants with BA (1,2). Currently, ~70–80% of patients with BA will eventually require liver transplantation, making this condition the leading indication for pediatric liver transplantation worldwide (3). Currently, the causes of this disease are largely undefined (4) and the underlying pathogenesis must be better understood.
Analyses of portal tracts and/or biliary remnants in the BA patients demonstrated that the extrahepatic bile duct was partially or entirely destroyed by residual inflammatory cells. The inflammatory milieu consists of Kupffer cells, CD4+ T cells, and natural killer (NK) lymphocytes; later, the CD68+ monocyte/macrophage population appears. These cells serve key roles in immune injury in mediating destruction of the extrahepatic and intrahepatic bile ducts and progression to hepatic cirrhosis (5).
Numerous studies of BA have presented clear evidence of the cytotoxicity of various lymphocytes in mediating immune-mediated ductal destruction (6). NK lymphocytes can initiate nonspecific cytotoxicity of epithelial lining and produce the obstructive phenotype typical of experimental BA (5,6,7). CD8+ cells can secondarily injure the epithelium following NK cell activity. In a mouse model of BA, neonatal cytotoxic CD8+ T lymphocytes can render the neonate more susceptible to obstruction of bile ducts. In addition, the loss of CD8+ lymphocytes prevents obstruction of the bile ducts and suppresses the disease phenotype (8). Further, a recent study suggested that autoreactive T lymphocytes can recognize bile duct epithelial cells (9). Although these previous reports have identified molecular and cellular effectors that regulate inflammation and obstruction of bile ducts, a characterization of the cytokine milieu, which is especially important in understanding the clinical immune pathogenesis of the disease, has not been completed.
In this study, the liver-infiltrating inflammatory environment and peripheral blood T cells were characterized to detect characteristic expansions of T lymphocytes and NK cells in the portal tracts of BA patients.
Results
Subject Characteristics
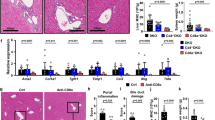
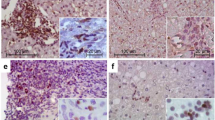
All patients with BA and the cholestatic controls (neonatal hepatitis and choledochal cysts) had conjugated hyperbilirubinemia and elevated serum γ-glutamyltransferase and alanine aminotransferase levels. Table 1 presents demographic data and serum bilirubin, γ-glutamyltransferase, and alanine aminotransferase values from each patient group. Liver tissue was analyzed from patients with BA and cholestatic disease. The presence of periductal inflammation was documented by hematoxylin and eosin staining of a portion of each tissue specimen collected, as shown in Figure 1 . Because the extrahepatic bile duct is the primary target in BA, histology of cholestasis from BA and liver disease other than BA revealed marked bile ductular proliferation with bile plugs, mixed inflammatory cellular infiltration, and varying degrees of portal tract fibrosis. Portal tracts were identified by characteristic vessel formation and bile duct epithelium and confirmed by cytokeratin-7 staining. Extensive periductal inflammation was observed within the portal tracts of BA patients. Liver tissue from cholestatic disease controls likewise had portal tract infiltrates ( Figure 1 ).
Identification of inflammation and bile duct hyperplasia in BA. Hematoxylin and eosin staining of transverse sections of liver biopsies from an infant with BA and an infant with TPN-related cholestasis. Marked inflammation and bile ductular proliferation within the portal tracts were observed in subjects with BA (a) and those with TPN-related cholestasis (b). Original magnification ×200. Bars = 50 µm. BA, biliary atresia; TPN, total parenteral nutrition.
Subtype and Distribution of Lymphocytes Within Extrahepatic Bile Ducts of Infants With BA
Previous studies have found that the inflammatory milieu present in the portal tracts of patients with BA consists of Kupffer cells, CD8+ T cells, and NK lymphocytes. To further explore the anatomical relationship between lymphocytes and bile ducts in the pathogenesis of human BA, we documented the phenotype of lymphocyte subtypes, neutrophils, and macrophages present in the extrahepatic portal tracts of patients with BA and control subjects. In this research, surface expression staining identified subtype mononuclear cell lineages, including T cells (CD3), and macrophage/Kupffer cells (CD68) in the bile ducts of BA patients. The cell-surface marker CD56 was used routinely to identify classic NK lymphocytes ( Figure 2a ).
The phenotype of lymphocyte subtypes, neutrophils, and macrophages in BA and control. (a) Liver sections were analyzed for the distribution of CD68, CD3, and CD56 in the portal tracts in BA by standard immunofluorescence techniques. Tissue sections from patients with cholestatic liver disease (A, B, C), and patients with BA (D, E, F) were incubated with goat anti-mouse IgG-fluorescein isothiocyanate (FITC) (green) and counterstained with nuclear stain DAPI (blue) to detect cellular nuclei. Representative patterns of CD68 (A, D), CD3 (B, E), CD56 (C, F) staining in the portal tracts of patients with BA and cholestatic controls are shown (original magnification ×100). Bars = 50 µm. (b) Total CD3+ lymphocyte (×106) yield from BA tissue culture determined with a hemocytometer showed that significantly higher yield of immune cells were detected in BA livers (black bars) and extrahepatic duct remnants (gray bars) as compared with controls (white bars). (c) Flow cytometric analysis depicting quantification of hepatic mononuclear cells in livers (upper panel) and extrahepatic ducts (lower panel) with BA. Summary of cell-surface expression of CD3, CD4, and CD8. The vertical axes show the average number of cells (×105) per liver ± SD. n = 3–4 for each group; *P < 0.05 when the BA group (gray bars) is compared with the control group (white bars). The horizontal axes show the surface markers identifying specific cell types. BA, biliary atresia; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride.
To directly determine the number and functional commitment of hepatic NK and T cells in bile ducts (including gallbladder tissue) in patients with BA, we further analyzed the cultured extrahepatic bile duct tissues by isolating mononuclear cells. Extrahepatic bile duct tissue from patients with BA yielded greater numbers of CD3+ immune cells as compared with control tissue ( Figure 2b ). Quantitative analysis of each type of immune cell in the portal tract tissue revealed that NK lymphocytes are the most common resident inflammatory cells populating extrahepatic bile ducts of both the controls and patients with BA. NK lymphocytes were increased eight- to 10-fold in patients with BA as compared with normal livers and those from the controls with cholestatic disease ( Figure 2c ). NK lymphocytes are the most common resident inflammatory cells in the neonatal extrahepatic bile duct.
We also cultured liver and extrahepatic bile duct remnant tissue samples to examine the preferential expansion of previously activated T cells. Subtypes of T lymphocytes were determined by staining for expression of CD3 (present on all T cells), CD4, and CD8. CD4+ T cells were the predominant phenotype and were present in BA and control duct tissues. Quantification of T-cell subtypes demonstrated that significantly more CD8+ cells were present in the BA liver tissue as compared with normal livers or other infantile cholestatic disease samples ( Figure 2c ) (P < 0.05). Anatomical distribution and quantification of NK lymphocytes and CD8 cells suggested that these cells play a key role in the pathogenesis of bile duct injury during postnatal organ development.
Expression of Costimulatory Molecules and Their Ligands, CD28/CTLA-4, in Extrahepatic Bile Ducts
We determined whether the anatomical relationship between lymphocytes and duct epithelial cells has functional relevance. To address this issue, hepatic mRNA transcripts from genes involved in T-cell activation, including those for immunologic costimulatory factors CD80 (B7-1), CD86 (B7-2), and CD40 and their ligands CD28/CTLA-4, were quantified in the patients with BA and control subjects using real-time reverse-transcriptase PCR. As shown in Figure 3 , the expression of all four genes related to T-cell activation and cytotoxicity increased 1.5- to ~150-fold in livers of infants with BA relative to controls ( Figure 3 ). Normal liver tissue, choledochal cysts, and neonatal hepatitis liver tissue samples did not show increased T-cell activation and cytotoxicity profiles, with very low expression of B7-1, B7-2, CD40, and CD28 and negative values for CTLA-4 expression (data not shown). Among these genes, the molecular signature represents a functional state, rather than a reflection of increased cell numbers in the portal tracts ( Figure 3 ). The mRNA expression studies clarified that T-cell activation and cytotoxicity were exclusive to BA and were not found in normal livers or the other two cholestatic liver diseases.
Striking expansion of genes encoding T-cell and NK activation markers in BA liver and extrahepatic duct remnants. RNA samples from the livers of patients with BA (n = 10) and normal controls (n = 4) were analyzed for the presence of cytokine-specific mRNA transcripts by RT-PCR. The mRNA fold changes of patients with BA are shown as compared with controls. BA, biliary atresia; NK, natural killer; RT-PCR, reverse-transcriptase PCR.
Recognizing that NK lymphocytes may also be implicated in pathogenesis of BA, we determined the expression of CD69, Nkg2d, granzyme B, and NKp46, which were involved in the NK cell cytotoxicity by quantitative reverse-transcriptase PCR analysis. As shown in Figure 3 , we observed striking overexpression, by several fold, of all four genes in the liver and/or bile duct remnant samples from patients with BA as compared with controls ( Figure 3 ). In contrast, CD69, Nkg2d, granzyme B, and NKp46 were not expressed in control livers or in extrahepatic bile ducts (data not shown).
Prominent Lymphocyte Populations of Extrahepatic Bile Ducts in Patients With BA and Controls
To gain further insight into which NK and T-cell populations may be more important for injuring duct epithelium and giving rise to the atresia phenotype, we determined whether hepatic NK and CD8+ cells could induce lysis in the human cholangiocyte line HIBEpiC. Although CD8+ cells from control liver tissues were unable to induce HIBEpiC lysis, CD8+ cells isolated from patients with BA could promote HIBEpiC lysis within 5 h of culture ( Figure 4a ). The cytolytic properties were specific to HIBEpiC cells, as supported by the lack of lysis of the K562 cell line in coculture with CD8+ cells from patients with BA.
Activity of hepatic NK and CD8+ T cells toward cholangiocyte lysis. Hepatic lymphocytes isolated from livers were subjected to coculture with NK lymphocytes at specific effector cell/target cell (E/T) ratios (gray bars, ratio 1:20; black bars, ratio 1:10; white bars, ratio 1:1) and appropriately stained. The percentage of cells positive for calcein acetoxymethyl ester plus ethidium homodimer-1 among the total number of cells positive (%) was calculated using flow cytometry. (a) NK lymphocytes; (b) CD8+ T cells. Results are representative of three independent experiments, with hepatic lymphocytes obtained from a pool of three to eight livers (*P < 0.01 when biliary atresia is compared with corresponding cholestatic control). NK, natural killer.
The cytotoxic capability of NK lymphocytes was further investigated based on the aforementioned cellular markers of NK cell activation and function. Although NK lymphocytes from the patients with BA also induced similar lysis of the HIBEpiC and K562 cell lines, NK lymphocytes from patients with BA showed significantly higher levels of lysis than those from control samples at an effector cell/target cell ratio of 20:1 ( Figure 4b , n = 6, P < 0.01). The cytotoxicities of the NK cells tested were near or less than those of CD8+ T cells. In contrast, NK lymphocytes from control liver tissues induced much less lysis in either the HIBEpiC or K562 cell lines.
Discussion
The role of the immune system in BA, which could lead to bile duct epithelial injury and eventual obstruction and fibrosis of the extrahepatic bile duct, has been proposed in the past (10,11). At the time of diagnosis of BA in humans, evidence of a mounting proinflammatory response and temporospatial features of bile duct injury can be observed. The portal tract inflammation in BA is an immune response to the presence of cholestasis, indicating that the role of autoimmunity in human BA should be a focus for studying the pathogenesis of this disease (12). It remains to be determined whether the atresia phenotype results from a hyperimmune response by NK or CD8+ cells that may be linked to the immaturity of counterregulatory circuits in the neonatal period. This study shows clear immune invasion within BA livers, consisting of Kupffer cells, CD4+ T cells, and CD8+ T cells with local expression of CD80 and CD86, demonstrating the generation of progressive bile duct injury with duct-specific molecular and cellular activity in infants with BA.
NK lymphocytes have been recognized as resident inflammatory cells and responders to infectious challenges in the liver (13). Virus-infected cells can downregulate major histocompatibility complex class I products in order to escape T-cell recognition, and NK proliferation and activation in the liver might destroy these virus-infected cells (14). In this research, we found that NK lymphocytes are abundant in neonatal extrahepatic bile ducts and increase in cell number or activity in duct obstruction of BA, including that caused by the presence of cytomegalovirus infection in patients with BA as compared with controls. In this study, the NKG2D and NKp46 receptors were also shown to be highly expressed, which could direct antiviral effects to infected cells by promotion of inflammatory responses. Furthermore, the liver NK lymphocytes were activated as evidenced by their abilities to lyse the human cholangiocyte cell line HIBEC. The activated NK lymphocytes isolated from patients with BA could lyse an uninfected cholangiocyte cell line, as reported here, suggesting that that these cells may have the potential to injure neighboring uninfected cholangiocytes in vivo, via the recognition of cellular epitopes by molecular mimicry (15,16).
Besides NK lymphocytes, other effector cells (such as CD8+ cells) may also be required to carry out epithelial injury and obstruct the duct lumen (17). In this study, we found that CD8+ T cells infiltrated within the extrahepatic bile duct remnants in 95% of the cases with BA, suggesting that cytotoxic CD8+ T cells could mediate inflammatory infiltration of the duct wall. A previous study reported that the loss of CD8+ cells minimized bile duct epithelial damage but was not sufficient to abolish the duct lumen obstruction. Rhesus rotavirus–primed (but not naive) hepatic CD8+ cells can promote lysis of naive cholangiocytes, probably in a second specific wave of epithelial injury (8,18). Consistent with this report, we found that CD8+ T-cell subsets could be isolated from BA tissue, and hepatic CD8+ cells from patients with BA could lyse a cholangiocyte cell line but did not damage the control cell line. These results are consistent with a temporal and spatial coordination of cellular events. These extend the contact-dependent injury of adjacent naive cholangiocytes and induce the adaptive immune system to generate a robust proinflammatory response in an autoimmune fashion (19).
Human costimulatory molecule (CD80 and CD86) gene products convey potent immunoactivating properties for CD8 cell-mediated cytotoxicity on target cells (20). Our results demonstrated a marked increase in CD80 and CD86 expression in BA. Furthermore, there was a close correlation between the expression of CD80/CD86 and CD8+ lymphocytes. We have demonstrated that only a few cells express the costimulatory molecules CD80 and CD86 in normal individuals, suggesting that the abundance of costimulatory molecules might be the basis for the activation of cytotoxic CD8 T cells. It is quite likely that in human BA, activators of T lymphocytes are upregulated, probably due to increased expression of CD80/CD86 and CD40 (21).
Collectively, these data support the existence of a biological setting in which either individually or in synergy, it can be inferred further that the lymphocytic infiltrate is primarily composed of NK and CD8+ T cells, and both neonatal NK and CD8+ cells could effectively mount innate and adaptive responses that target the site of infection and emerge as cellular effectors of luminal obstruction. These cells ultimately produce a phenotype akin to a human disease in young infants. The potential role of NK and T cells in bile duct injury was suggested previously by transplantation of CD3+ cells (probably containing CD4+, CD8+, and NK cell subpopulations) into adult severe combined immunodeficiency mice. Therefore, NK and CD8+ T cells constitute two complementary mechanisms that act in concert to produce the atresia phenotype (6).
This study describes a unique inflammatory infiltration profile seen in BA as compared with controls, supporting the role of specific immune destruction of bile ducts involved in the pathogenesis of BA. Further elucidation of the nature and the source of inflammatory infiltration, such as specific antigen (e.g., viral or self–bile duct epithelial antigen), will be essential.
In summary, these findings underscore the role of the developing immune system in the pathogenesis of BA and offer strong evidence that the synergy of a very large number of activated CD8+ T cells and NK lymphocytes mediate the pathogenesis of biliary obstruction not present in other similar neonatal cholestatic disorders. Determining the nature of these activated NK and CD8 cells is essential for understanding this disorder and for identifying a potential therapeutic target for effective treatment of this disease.
Methods
Study Subjects and Sample Collection
Patients were consecutively recruited from January 2006 to July 2009 at the Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China, with detailed clinical data. A flow chart illustrates which tissue samples were analyzed using which method in which patient group ( Figure 5 ). All subjects were ethnic Chinese. This study was approved by the institutional review board of the Chongqing Medical University, Chongqing, People’s Republic of China, and written informed consent was obtained from the patients’ guardians. The study consisted of 16 patients with pathologically confirmed BA based on resected biliary remnants during a Kasai portoenterostomy (n = 16). Control groups with comparable age to patients with BA included patients with cholestatic disease other than BA, such as total parenteral nutrition–related cholestatic disease, choledochal cysts, or neonatal hepatitis (which requires an intraoperative cholangiogram to rule out BA) (cholestatic controls, n = 9) and noncholestatic controls (normal, n = 3). Cholestatic disease patients were chosen because of clinical and histologic similarities to BA. Noncholestatic (normal) controls included three percutaneous liver biopsies under general anesthetic. Peripheral blood mononuclear cells, fresh liver wedge tissue, and extrahepatic bile duct remnant tissue were collected from 16 patients with BA before or at the time of the Kasai portoenterostomy. Tissue was processed for histological evaluation by light microscopy to confirm the diagnosis of BA. Patients with BA underwent serial blood sampling preoperatively and at 1 wk and 1, 3, and 6 mo after Kasai portoenterostomy. Samples were obtained at routine clinic visits, and patients were otherwise well. Liver wedge tissue was collected from all cases and controls.
Immunofluorescence Staining and Histopathology
Paraffin-embedded or cryopreserved sections of livers and extrahepatic bile ducts were obtained and examined. Primary antibodies were incubated with sections overnight at 4 °C using optimal dilutions. Mouse antihuman CD56 antibody (BD Biosciences, San Jose, CA) was used to stain NK lymphocytes, antihuman CD3 antibody (BD Biosciences) to stain T cells, and antihuman cytokeratin-7 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) to stain cholangiocytes. After washing with phosphate-buffered saline, slides were incubated with the appropriate affinity-purified fluorescein isothiocyanate– or rhodamine-conjugated secondary antibodies (Jackson Immuno Research, West Grove, PA) for 30 min. Appropriate positive and negative control slides were processed in parallel with study slides, including an isotype-matched immunoglobulin at the same concentration as that of the primary antibody. Tissues were then counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride and sealed with VectaShield (Vector Laboratories, Burlingame, CA). Slides were visualized using the Leica DMRA2 microscope (Leica Microsystems, Bannockburn, IL). Portal tracts and selected hepatic lobule areas for each antibody experiment were photographed. Spectral unmixing was accomplished using Nuance software v1.42 (Cambridge Research, Woburn, MA) with pure spectral libraries of individual chromogens. Images were collected using a digital camera (Nikon) and imported into Adobe Photoshop (Adobe Systems). For quantification, 20 high-power fields were examined for each tissue slide. All slides were independently analyzed by two blinded researchers.
Isolation of Mononuclear Cells From Tissue
Liver and extrahepatic bile duct tissues were gently minced, passed through a 40-µm cell strainer, and centrifuged at 270g, 4 °C, two times. Mononuclear cells were resuspended in RPMI 1640 media (Invitrogen, Carlsbad, CA). Mononuclear cells isolated from livers or extrahepatic bile ducts were analyzed by two-color immunofluorescence using monoclonal antibodies. Specific cell-surface markers were used to perform cellular phenotyping, including fluorescein isothiocyanate– or phycoerythin-conjugated anti-CD56 (NK lymphocytes), anti-CD68 (macrophages/Kupffer cells), anti-CD3 (T cells), anti-CD4 (subtype of T-cells), anti-CD8a (subtype of T-cells), and anti-CD19 (B cells). These antibodies were purchased from BD Biosciences. Flow cytometry was completed with a FACSCalibur cytometer and analyzed using CellQuest software (BD Biosciences). Cell populations were selected according to forward/side scatter, gated according to isotype controls to account for background fluorescence, and subjected to secondary analysis based on fluorescence signals by individual antibodies. Median fluorescence intensity was determined according to the costaining of selected cytokines. For histograms, gates were established using signals from cell-surface markers.
Gene Expression
Cellular RNA was isolated from liver and extrahepatic bile duct samples from patients with BA or controls using Trizol (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. cDNA was synthesized with 2 µg of RNA using the Advantage RT for PCR kit (Clontech, Mountain View, CA). RNA integrity was verified by 260:280 ratios between 1.8 and 2.2. Entire RNA samples were then treated with deoxyribonuclease 1 (Sigma Chemical, St. Louis, MO) to remove any contaminating genomic DNA. A lack of DNA contamination was verified by eliminating reverse transcriptase during cDNA synthesis in control samples. Primers specific for CD80, CD86, CD40, CD28, CD69, NKG2D, NKp46, and Granzyme B are listed in Table 2 . Real-time PCR was performed with SYBR Green (Roche, Shanghai, China) using the ABI PRISM 7700 Sequence Detection System (Applied BioSystems, Foster City, CA). All samples were normalized to the signal generated from a housekeeping gene, β-actin. Transcript levels were calculated using the double delta Ct equation (Applied Biosystems, Carlsbad, CA). Results are reported as the fold change in mRNA expression of patients with BA as compared with controls.
NK or CD8 Cell Cytotoxicity
Peripheral blood mononuclear cells were isolated from blood samples of patients with BA and control patients by separation on a Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) gradient and resuspended in fluorescence-activated cell sorter buffer for analysis. Primary human CD8+ T lymphocytes were purified from peripheral blood mononuclear cells by magnetic-activated cell sorting using the BioMag Human CD8+ T cell Enrichment System (Bangs Laboratories, Fishers, IN). NK lymphocytes were enriched from hepatic mononuclear cells (isolated from liver and extrahepatic bile duct tissue) using an NK cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). The cells were counted and tested for viability by trypan blue exclusion. Purity was confirmed to exceed 95% by flow cytometry.
The assay of NK cell– or CD8+ T cell–induced lysis of cholangiocytes was measured with a Live/Dead viability/cytotoxicity kit for mammalian cells (Invitrogen) in various effector/target ratios as described previously. Briefly, cells of the human cholangiocyte cell line HIBEC (target cells) were stained with calcein acetoxymethyl ester and then cocultured for 3 h with NK or CD8 cells at specific effector cell/target cell ratios. After incubation, the cells were stained with ethidium homodimer-1. Cytotoxicity was analyzed by flow cytometry and calculated as the percentage of cells positive for calcein acetoxymethyl ester plus ethidium homodimer-1 among the total number of cells positive for calcein acetoxymethyl ester. Assays were performed at the indicated effector cell/target cell ratios. The K562 cell line was also used in cytolytic assays as a control. Assays were performed in triplicate wells, and a response was considered positive if specific lysis was at least twice the value of the negative control.
Statement of Financial Support
The research was supported by the National Natural Science Foundation of China (grant nos. 30973440 and 30770950) and was a key project of the Chongqing Natural Science Foundation (CSTC, 2008BA0021).
References
Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH . Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007;46:566–81.
Bezerra JA, Tiao G, Ryckman FC, et al. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet 2002;360:1653–9.
Mack CL . The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis 2007;27:233–42.
Barnes BH, Tucker RM, Wehrmann F, Mack DG, Ueno Y, Mack CL . Cholangiocytes as immune modulators in rotavirus-induced murine biliary atresia. Liver Int 2009;29:1253–61.
Shivakumar P, Sabla GE, Whitington P, Chougnet CA, Bezerra JA . Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest 2009;119:2281–90.
Mack CL, Tucker RM, Lu BR, et al. Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia. Hepatology 2006;44:1231–9.
Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest 2004;114:322–9.
Shivakumar P, Sabla G, Mohanty S, et al. Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology 2007;133:268–77.
Mack CL, Falta MT, Sullivan AK, et al. Oligoclonal expansions of CD4+ and CD8+ T-cells in the target organ of patients with biliary atresia. Gastroenterology 2007;133:278–87.
Miethke AG, Saxena V, Shivakumar P, Sabla GE, Simmons J, Chougnet CA . Post-natal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J Hepatol 2010;52:718–26.
Erickson N, Mohanty SK, Shivakumar P, Sabla G, Chakraborty R, Bezerra JA . Temporal-spatial activation of apoptosis and epithelial injury in murine experimental biliary atresia. Hepatology 2008;47:1567–77.
Mack CL, Tucker RM, Sokol RJ, Kotzin BL . Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol 2005;115:200–9.
Kim KD, Zhao J, Auh S, et al. Adaptive immune cells temper initial innate responses. Nat Med 2007;13:1248–52.
Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S . Functions of natural killer cells. Nat Immunol 2008;9:503–10.
Ogasawara K, Hamerman JA, Hsin H, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity 2003;18:41–51.
Arenz M, Pingel S, Schirmacher P, Meyer zum Büschenfelde KH, Löhr HF . T cell receptor Vbeta chain restriction and preferred CDR3 motifs of liver-kidney microsomal antigen (LKM-1)-reactive T cells from autoimmune hepatitis patients. Liver 2001;21:18–25.
Buxbaum J, Qian P, Khuu C, et al. Novel model of antigen-specific induction of bile duct injury. Gastroenterology 2006;131:1899–906.
Mack CL, Tucker RM, Sokol RJ, et al. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pediatr Res 2004;56:79–87.
Jafri M, Donnelly B, Allen S, et al. Cholangiocyte expression of alpha2beta1-integrin confers susceptibility to rotavirus-induced experimental biliary atresia. Am J Physiol Gastrointest Liver Physiol 2008;295:G16–26.
Leifeld L, Trautwein C, Dumoulin FL, Manns MP, Sauerbruch T, Spengler U . Enhanced expression of CD80 (B7-1), CD86 (B7-2), and CD40 and their ligands CD28 and CD154 in fulminant hepatic failure. Am J Pathol 1999;154:1711–20.
Wang P, Liu Z, Wu C, Zhu B, Wang Y, Xu H . Evaluation of CD86/CD28 and CD40/CD154 pathways in regulating monocyte-derived CD80 expression during their interaction with allogeneic endothelium. Transplant Proc 2008;40:2729–33.
Acknowledgements
The authors express gratitude to Kaiyong Tang for providing technical assistance and insightful discussions during the preparation of the manuscript and without whom this work would not have been possible. We thank Xiaoyong Zhang, at the Wistar Institute, USA, for help with the linguistic revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, C., Zhu, J., Pu, CL. et al. Combinatory effects of hepatic CD8+ and NK lymphocytes in bile duct injury from biliary atresia. Pediatr Res 71, 638–644 (2012). https://doi.org/10.1038/pr.2012.17
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.17
This article is cited by
-
Expression of programmed death-1 and its ligands in the liver of biliary atresia
World Journal of Pediatrics (2017)