Abstract
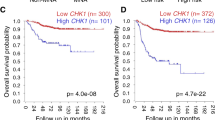
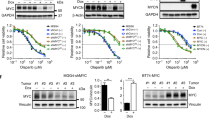
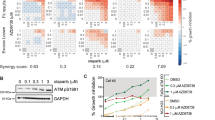
High-risk and MYCN-amplified neuroblastomas are among the most aggressive pediatric tumors. Despite intense multimodality therapies, about 50% of these patients succumb to their disease, making the search for effective therapies an absolute priority. Due to the important functions of poly (ADP-ribose) polymerases, PARP inhibitors have entered the clinical settings for cancer treatment and are being exploited in a variety of preclinical studies and clinical trials. PARP inhibitors based combination schemes have also been tested in neuroblastoma preclinical models with encouraging results. However, the expression of PARP enzymes in human neuroblastoma and the biological consequences of their inhibition remained largely unexplored. Here, we show that high PARP1 and PARP2 expression is significantly associated with high-risk neuroblastoma cases and poor survival, highlighting its previously unrecognized prognostic value for human neuroblastoma. In vitro, PARP1 and 2 are abundant in MYCN amplified and MYCN-overexpressing cells. In this context, PARP inhibitors with high ‘PARP trapping’ potency, such as olaparib or talazoparib, yield DNA damage and cell death preceded by intense signs of replication stress. Notwithstanding the activation of a CHK1-CDC25A replication stress response, PARP-inhibited MYCN amplified and overexpressing cells fail to sustain a prolonged checkpoint and progress through mitosis in the presence of damaged DNA, eventually undergoing mitotic catastrophe. CHK1-targeted inhibition of the replication stress checkpoint exacerbated this phenotype. These data highlight a novel route for cell death induction by PARP inhibitors and support their introduction, together with CHK1 inhibitors, in therapeutic approaches for neuroblastomas with high MYC(N) activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009; 27: 289–297.
Bagatell R, Beck-Popovic M, London WB, Zhang Y, Pearson AD, Matthay KK et al. Significance of MYCN amplification in international neuroblastoma staging system stage 1 and 2 neuroblastoma: a report from the International Neuroblastoma Risk Group database. J Clin Oncol 2009; 27: 365–370.
Canete A, Gerrard M, Rubie H, Castel V, Di Cataldo A, Munzer C et al. Poor survival for infants with MYCN-amplified metastatic neuroblastoma despite intensified treatment: the International Society of Paediatric Oncology European Neuroblastoma Experience. J Clin Oncol 2009; 27: 1014–1019.
Valentijn LJ, Koster J, Haneveld F, Aissa RA, van Sluis P, Broekmans ME et al. Functional MYCN signature predicts outcome of neuroblastoma irrespective of MYCN amplification. Proc Natl Acad Sci USA 2012; 109: 19190–19195.
Westermann F, Muth D, Benner A, Bauer T, Henrich KO, Oberthuer A et al. Distinct transcriptional MYCN/c-MYC activities are associated with spontaneous regression or malignant progression in neuroblastomas. Genome Biol 2008; 9: R150.
Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM . Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J 1997; 16: 2985–2995.
Ame JC, Spenlehauer C, de Murcia G . The PARP superfamily. BioEssays 2004; 26: 882–893.
Li M, Yu X . The role of poly(ADP-ribosyl)ation in DNA damage response and cancer chemotherapy. Oncogene 2014; 34: 3349–3356.
Lupo B, Trusolino L . Inhibition of poly(ADP-ribosyl)ation in cancer: old and new paradigms revisited. Biochim Biophys Acta 2014; 1846: 201–215.
Feng FY, de Bono JS, Rubin MA, Knudsen KE . Chromatin to clinic: the molecular rationale for PARP1 inhibitor function. Mol Cell 2015; 58: 925–934.
Helleday T . The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol 2011; 5: 387–393.
Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res 2012; 72: 5588–5599.
Durkacz BW, Omidiji O, Gray DA, Shall S . (ADP-ribose)n participates in DNA excision repair. Nature 1980; 283: 593–596.
Sonnenblick A, de Azambuja E, Azim HA Jr., Piccart M . An update on PARP inhibitors-moving to the adjuvant setting. Nat Rev Clin Oncol 2015; 12: 27–41.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005; 434: 913–917.
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434: 917–921.
Daniel RA, Rozanska AL, Thomas HD, Mulligan EA, Drew Y, Castelbuono DJ et al. Inhibition of poly(ADP-ribose) polymerase-1 enhances temozolomide and topotecan activity against childhood neuroblastoma. Clin Cancer Res 2009; 15: 1241–1249.
McCluskey AG, Mairs RJ, Tesson M, Pimlott SL, Babich JW, Gaze MN et al. Inhibition of poly(ADP-Ribose) polymerase enhances the toxicity of 131I-metaiodobenzylguanidine/topotecan combination therapy to cells and xenografts that express the noradrenaline transporter. J Nucl Med 2012; 53: 1146–1154.
McNeil EM, Ritchie AM, Melton DW . The toxicity of nitrofuran compounds on melanoma and neuroblastoma cells is enhanced by Olaparib and ameliorated by melanin pigment. DNA Repair 2013; 12: 1000–1006.
Mueller S, Bhargava S, Molinaro AM, Yang X, Kolkowitz I, Olow A et al. Poly (ADP-Ribose) polymerase inhibitor MK-4827 together with radiation as a novel therapy for metastatic neuroblastoma. Anticancer Res 2013; 33: 755–762.
Norris RE, Adamson PC, Nguyen VT, Fox E . Preclinical evaluation of the PARP inhibitor, olaparib, in combination with cytotoxic chemotherapy in pediatric solid tumors. Pediatr Blood Cancer 2014; 61: 145–150.
Judware R, Culp LA . Over-expression of transfected N-myc oncogene in human SKNSH neuroblastoma cells down-regulates expression of beta 1 integrin subunit. Oncogene 1995; 11: 2599–2607.
Lutz W, Stohr M, Schurmann J, Wenzel A, Lohr A, Schwab M . Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene 1996; 13: 803–812.
Utani K, Kohno Y, Okamoto A, Shimizu N . Emergence of micronuclei and their effects on the fate of cells under replication stress. PloS One 2010; 5: e10089.
Lukas C, Savic V, Bekker-Jensen S, Doil C, Neumann B, Pedersen RS et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol 2011; 13: 243–253.
Zellweger R, Dalcher D, Mutreja K, Berti M, Schmid JA, Herrador R et al. Rad51-mediated replication fork reversal is a global response to genotoxic treatments in human cells. J Cell Biol 2015; 208: 563–579.
Ray Chaudhuri A, Hashimoto Y, Herrador R, Neelsen KJ, Fachinetti D, Bermejo R et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat Struct Mol Biol 2012; 19: 417–423.
Berti M, Ray Chaudhuri A, Thangavel S, Gomathinayagam S, Kenig S, Vujanovic M et al. Human RECQ1 promotes restart of replication forks reversed by DNA topoisomerase I inhibition. Nat Struct Mol Biol 2013; 20: 347–354.
Bryant HE, Petermann E, Schultz N, Jemth AS, Loseva O, Issaeva N et al. PARP is activated at stalled forks to mediate Mre11-dependent replication restart and recombination. EMBO J 2009; 28: 2601–2615.
Petroni M, Veschi V, Prodosmo A, Rinaldo C, Massimi I, Carbonari M et al. MYCN sensitizes human neuroblastoma to apoptosis by HIPK2 activation through a DNA damage response. Mol Cancer Res 2011; 9: 67–77.
Petroni M, Sardina F, Heil C, Sahun-Roncero M, Colicchia V, Veschi V et al. The MRN complex is transcriptionally regulated by MYCN during neural cell proliferation to control replication stress. Cell Death Differ 2016; 23: 197–206.
Ben-Yosef T, Yanuka O, Halle D, Benvenisty N . Involvement of Myc targets in c-myc and N-myc induced human tumors. Oncogene 1998; 17: 165–171.
Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther 2014; 13: 433–443.
Murai J, Zhang Y, Morris J, Ji J, Takeda S, Doroshow JH et al. Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition. J Pharmacol Exp Ther 2014; 349: 408–416.
Dominguez-Sola D, Ying CY, Grandori C, Ruggiero L, Chen B, Li M et al. Non-transcriptional control of DNA replication by c-Myc. Nature 2007; 448: 445–451.
Felsher DW, Bishop JM . Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc Natl Acad Sci USA 1999; 96: 3940–3944.
Kuzyk A, Mai S . c-MYC-induced genomic instability. Cold Spring Harb Perspect Med 2014; 4: a014373.
Neiman PE, Kimmel R, Icreverzi A, Elsaesser K, Bowers SJ, Burnside J et al. Genomic instability during Myc-induced lymphomagenesis in the bursa of Fabricius. Oncogene 2006; 25: 6325–6335.
Ray S, Atkuri KR, Deb-Basu D, Adler AS, Chang HY, Herzenberg LA et al. MYC can induce DNA breaks in vivo and in vitro independent of reactive oxygen species. Cancer Res 2006; 66: 6598–6605.
Rohban S, Campaner S . Myc induced replicative stress response: How to cope with it and exploit it. Biochim Biophys Acta 2015; 1849: 517–524.
Srinivasan SV, Dominguez-Sola D, Wang LC, Hyrien O, Gautier J . Cdc45 is a critical effector of myc-dependent DNA replication stress. Cell Rep 2013; 3: 1629–1639.
Neelsen KJ, Zanini IM, Herrador R, Lopes M . Oncogenes induce genotoxic stress by mitotic processing of unusual replication intermediates. J Cell Biol 2013; 200: 699–708.
Chayka O, D'Acunto CW, Middleton O, Arab M, Sala A . Identification and pharmacological inactivation of the MYCN gene network as a therapeutic strategy for neuroblastic tumor cells. J Biol Chem 2015; 290: 2198–2212.
Cole KA, Huggins J, Laquaglia M, Hulderman CE, Russell MR, Bosse K et al. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. Proc Natl Acad Sci USA 2011; 108: 3336–3341.
Murga M, Campaner S, Lopez-Contreras AJ, Toledo LI, Soria R, Montana MF et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat Struct Mol Biol 2011; 18: 1331–1335.
Veschi V, Petroni M, Cardinali B, Dominici C, Screpanti I, Frati L et al. Galectin-3 impairment of MYCN-dependent apoptosis-sensitive phenotype is antagonized by nutlin-3 in neuroblastoma cells. PloS One 2012; 7: e49139.
Massimi I, Guerrieri F, Petroni M, Veschi V, Truffa S, Screpanti I et al. The HMGA1 protoncogene frequently deregulated in cancer is a transcriptional target of E2F1. Mol Carcinog 2013; 52: 526–534.
Petroni M, Veschi V, Gulino A, Giannini G . Molecular mechanisms of MYCN-dependent apoptosis and the MDM2-p53 pathway: an Achille's heel to be exploited for the therapy of MYCN-amplified neuroblastoma. Front Oncol 2012; 2: 141.
Veschi V, Petroni M, Bartolazzi A, Altavista P, Dominici C, Capalbo C et al. Galectin-3 is a marker of favorable prognosis and a biologically relevant molecule in neuroblastic tumors. Cell Death Dis 2014; 5: e1100.
Carbonari M . New use for an old reagent: Cell cycle analysis of DNA content using flow cytometry in formamide treated cells. Cytometry A 2016; 89: 498–503.
Ferrao PT, Bukczynska EP, Johnstone RW, McArthur GA . Efficacy of CHK inhibitors as single agents in MYC-driven lymphoma cells. Oncogene 2012; 31: 1661–1672.
Acknowledgements
Time-lapse experiments were performed at the Nikon Reference Center for Central-Southern Italy at IBPM-CNR. Financial Support: This work was supported by grants from: Associazione Italiana per la Ricerca sul Cancro IG17734 (G. Giannini), IG14723 (A. Gulino) and IG14535 (P. Lavia); AIRC MFAG13350 (G. Guarguaglini); AIRC 5XMILLE (A. Gulino); Ministry of University and Research (FIRB and PRIN projects) (A. Gulino and G. Giannini); Italian Institute of Technology (A. Gulino). M.P. has been recipient of a FIRC fellowship David Raffaelli.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Colicchia, V., Petroni, M., Guarguaglini, G. et al. PARP inhibitors enhance replication stress and cause mitotic catastrophe in MYCN-dependent neuroblastoma. Oncogene 36, 4682–4691 (2017). https://doi.org/10.1038/onc.2017.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.40
This article is cited by
-
DNA as the main target in radiotherapy—a historical overview from first isolation to anti-tumour immune response
Strahlentherapie und Onkologie (2023)
-
Class I HDAC inhibition reduces DNA damage repair capacity of MYC-amplified medulloblastoma cells
Journal of Neuro-Oncology (2023)
-
Combination bromo- and extraterminal domain and poly (ADP-ribose) polymerase inhibition synergistically enhances DNA damage and inhibits neuroblastoma tumorigenesis
Discover Oncology (2022)
-
Exploring the DNA damage response pathway for synthetic lethality
Genome Instability & Disease (2022)
-
Targeting autophagy reverses de novo resistance in homologous recombination repair proficient breast cancers to PARP inhibition
British Journal of Cancer (2021)