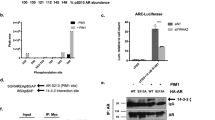
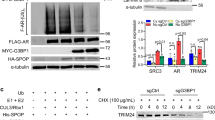
Abstract
Androgen receptor (AR) activation is critical for prostate cancer (PCa) development and progression, including castration resistance. The nuclear export signal of AR (NESAR) has an important role in AR intracellular trafficking and proteasome-dependent degradation. Here, we identified the RNA helicase DHX15 as a novel AR co-activator using a yeast mutagenesis screen and revealed that DHX15 regulates AR activity by modulating E3 ligase Siah2-mediated AR ubiquitination independent of its ATPase activity. DHX15 and Siah2 form a complex with AR, through NESAR. DHX15 stabilized Siah2 and enhanced its E3 ubiquitin-ligase activity, resulting in AR activation. Importantly, DHX15 was upregulated in PCa specimens and its expression was correlated with Gleason scores and prostate-specific antigen recurrence. Furthermore, DHX15 immunostaining correlated with Siah2. Finally, DHX15 knockdown inhibited the growth of C4-2 prostate tumor xenografts in mice. Collectively, our data argue that DHX15 enhances AR transcriptional activity and contributes to PCa progression through Siah2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30.
Hellerstedt BA, Pienta KJP . The cturrent state of hormonal therapy for prostate cancer. CA Cancer J Clin 2002; 52: 154–179.
Chan SC, Dehm SM . Constitutive activity of the androgen receptor. Adv Pharmacol 2014; 70: 327–366.
Kobayashi T, Inoue T, Kamba T, Ogawa O . Experimental evidence of persistent androgen-receptor-dependency in castration-resistant prostate cancer. Int J Mol Sci 2013; 14: 15615–15635.
Gelmann EP . Molecular biology of the androgen receptor. J Clin Oncol 2002; 20: 3001–3015.
Lonergan PE, Tindall DJ . Androgen receptor signaling in prostate cancer development and progression. J Carcinog 2011; 10: 20.
Picard D, Yamamoto KR . Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J 1987; 6: 3333–3340.
Poukka H, Karvonen U, Yoshikawa N, Tanaka H, Palvimo JJ, Janne OA . The RING finger protein SNURF modulates nuclear trafficking of the androgen receptor. J Cell Sci 2000; 113: 2991–3001.
Saporita AJ, Zhang Q, Navai N, Dincer Z, Hahn J, Cai X et al. Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. J Biol Chem 2003; 278: 41998–42005.
Zhou ZX, Sar M, Simental JA, Lane MV, Wilson EM . A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J Biol Chem 1994; 269: 13115–13123.
Gong Y, Wang D, Dar JA, Singh P, Graham L, Liu W et al. Nuclear export signal of androgen receptor (NESAR) regulation of androgen receptor level in human prostate cell lines via ubiquitination and proteasome-dependent degradation. Endocrinology 2012; 153: 5716–5725.
Nguyen MM, Harmon RM, Wang Z . Characterization of karyopherins in androgen receptor intracellular trafficking in the yeast model. Int J Clin Exp Pathol 2014; 7: 2768–2779.
Ferraldeschi R, Welti J, Luo J, Attard G, de Bono JS . Targeting the androgen receptor pathway in castration-resistant prostate cancer: progresses and prospects. Oncogene 2015; 34: 1745–1757.
Shafi AA, Yen AE, Weigel NL . Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther 2013; 140: 223–238.
Reed JC, Ely KR . Degrading liaisons: Siah structure revealed. Nat Struct Biol 2002; 9: 8–10.
Fukuba H, Takahashi T, Jin HG, Kohriyama T, Matsumoto M . Abundance of aspargynyl-hydroxylase FIH is regulated by Siah-1 under normoxic conditions. Neurosci Lett 2008; 433: 209–214.
Matsuzawa SI, Reed JC . Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell 2001; 7: 915–926.
Nadeau RJ, Toher JL, Yang X, Kovalenko D, Friesel R . Regulation of Sprouty2 stability by mammalian Seven-in-Absentia homolog 2. J Cell Biochem 2007; 100: 151–160.
Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell 2004; 117: 941–952.
Zhang J, Guenther MG, Carthew RW, Lazar MA . Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Development 1998; 12: 1775–1780.
Nakayama K, Qi J, Ronai Z . The ubiquitin ligase Siah2 and the hypoxia response. Mol Cancer Res 2009; 7: 443–451.
Qi J, Tripathi M, Mishra R, Sahgal N, Fazli L, Ettinger S et al. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell 2013; 23: 332–346.
Freeman MR . The ubiquitin ligase Siah2 is revealed as an accomplice of the androgen receptor in castration resistant prostate cancer. Asian J Androl 2013; 15: 447–448.
Arenas JE, Abelson JN . Prp43: An RNA helicase-like factor involved in spliceosome disassembly. Proc Nat Acad Sci USA 1997; 94: 11798–11802.
Combs DJ, Nagel RJ, Ares M Jr, Stevens SW . Prp43p is a DEAH-box spliceosome disassembly factor essential for ribosome biogenesis. Mol Cell Biol 2006; 26: 523–534.
Tanaka N, Aronova A, Schwer B . Ntr1 activates the Prp43 helicase to trigger release of lariat-intron from the spliceosome. Genes Dev 2007; 21: 2312–2325.
Fourmann JB, Schmitzova J, Christian H, Urlaub H, Ficner R, Boon KL et al. Dissection of the factor requirements for spliceosome disassembly and the elucidation of its dissociation products using a purified splicing system. Genes Dev 2013; 27: 413–428.
Koodathingal P, Novak T, Piccirilli JA, Staley JP . The DEAH box ATPases Prp16 and Prp43 cooperate to proofread 5' splice site cleavage during pre-mRNA splicing. Mol Cell 2010; 39: 385–395.
Walbott H, Mouffok S, Capeyrou R, Lebaron S, Humbert O, van Tilbeurgh H et al. Prp43p contains a processive helicase structural architecture with a specific regulatory domain. EMBO J 2010; 29: 2194–2204.
Brown JD, Beggs JD . Roles of PRP8 protein in the assembly of splicing complexes. EMBO J 1992; 11: 3721–3729.
Mosallanejad K, Sekine Y, Ishikura-Kinoshita S, Kumagai K, Nagano T, Matsuzawa A et al. The DEAH-box RNA helicase DHX15 activates NF-kappaB and MAPK signaling downstream of MAVS during antiviral responses. Sci Signal 2014; 7: ra40.
Coffey K, Robson CN . Regulation of the androgen receptor by post-translational modifications. J Endocrinol 2012; 215: 221–237.
Chymkowitch P, Le May N, Charneau P, Compe E, Egly JM . The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process. EMBO J 2011; 30: 468–479.
Gaughan L, Logan IR, Neal DE, Robson CN . Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res 2005; 33: 13–26.
Xu K, Shimelis H, Linn DE, Jiang R, Yang X, Sun F et al. Regulation of androgen receptor transcriptional activity and specificity by RNF6-induced ubiquitination. Cancer Cell 2009; 15: 270–282.
Li B, Lu W, Chen Z . Regulation of androgen receptor by E3 ubiquitin ligases: for more or less. Receptors Clin Investig 2014; 1; doi:10.14800%2Frci.122.
Hu G, Fearon ER . Siah-1 N-terminal RING domain is required for proteolysis function, and C-terminal sequences regulate oligomerization and binding to target proteins. Mol Cell Biol 1999; 19: 724–732.
Lorick KL, Jensen JP, Fang S, Ong AM, Hatakeyama S, Weissman AM . RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci USA 1999; 96: 11364–11369.
Scortegagna M, Subtil T, Qi J, Kim H, Zhao W, Gu W et al. USP13 enzyme regulates Siah2 ligase stability and activity via noncatalytic ubiquitin-binding domains. J Biol Chem 2011; 286: 27333–27341.
Clark EL, Coulson A, Dalgliesh C, Rajan P, Nicol SM, Fleming S et al. The RNA helicase p68 is a novel androgen receptor coactivator involved in splicing and is overexpressed in prostate cancer. Cancer Res 2008; 68: 7938–7946.
Fouraux MA, Kolkman MJ, Van der Heijden A, De Jong AS, Van Venrooij WJ, Pruijn GJ . The human La (SS-B) autoantigen interacts with DDX15/hPrp43, a putative DEAH-box RNA helicase. RNA 2002; 8: 1428–1443.
Grosso AR, Martins S, Carmo-Fonseca M . The emerging role of splicing factors in cancer. EMBO Rep 2008; 9: 1087–1093.
Dong X, Sweet J, Challis JR, Brown T, Lye SJ . Transcriptional activity of androgen receptor is modulated by two RNA splicing factors, PSF and p54nrb. Mol Cell Biol 2007; 27: 4863–4875.
Rajan P, Gaughan L, Dalgliesh C, El-Sherif A, Robson CN, Leung HY et al. The RNA-binding and adaptor protein Sam68 modulates signal-dependent splicing and transcriptional activity of the androgen receptor. J Pathol 2008; 215: 67–77.
Sequoia Ecosystem and Recreation Preserve Act of 1999. 106th Congress ed, US House of Representatives1999.
Haider AS, Kaye G, Thomson A . Autoimmune hepatitis in a demographically isolated area of Australia. Int MedJ 2010; 40: 281–285.
Zhao HL, Ueki N, Hayman MJ . The Ski protein negatively regulates Siah2-mediated HDAC3 degradation. Biochem Biophys Res Commun 2010; 399: 623–628.
Acknowledgements
We thank Aiyuan Zhang for technical support, Richard Gaber (Northwestern University) for advice on yeast screen, the PCBN for TMA with PCa specimens, Megan Lambert, Krystal Roskov and Robin Frederick for animal husbandry, and members of the Wang Lab for discussion. This work was supported in part by NIH R01 CA186780 (ZW), NIH R01 CA108675 (ZW), ACS #PF-05-229-01-CSM (MMN), the Department of Defense Prostate Cancer Research Program Award No W81XWH-14-2-0182, W81XWH-14-2-0183, W81XWH-14-2-0185, W81XWH-14-2-0186, and W81XWH-15-2-0062 PCBN, National Nature Science Foundation of China #81402091 (YJ), and National Natural Science Foundation of China Key Project 81130046 (JZ) and 2013GXNSFEA053004 (JZ). This work was also supported by a post-doctoral fellowship from the Urology Care Foundation of the American Urological Association (DW), the Mellam Family Fellowship (DW and KZM), the Tippens Scholarship (LEP) and NIH R50 CA211242-01 (LEP). In addition, this research was supported by the UPCI Animal Facility, Vector Core and Tissue and Research Pathology Services (TARPS) funded through NCI CCSG P30CA047904.
Author contributions
Yifeng Jing conceived and performed the majority of experiments and wrote manuscript. Minh M Nguyen performed the yeast screening. Dan Wang constructed the plasmids of flag-DHX15 and myc-DHX15, performed radioactive ligand-binding assay and ChIP and assisted with technical details and experimental design. Laura E Pascal performed animal experiments and analysis of the animal data, and helped with manuscript. Wenhuan Guo constructed the truncated Siah2 plasmids, performed parts of BrdU assay and several extractions of proteins and RNAs. Yadong Xu generated data for Supplementary Figures S1 and S3. Junkui Ai constructed the plasmid of Flag-AR and C4-2-AR, assisted with technical details. Fang-ming Deng performed IHC. Khalid Masoodi constructed and standardized the primers of DHX15. Xinpei Yu performed some of the ubiquitination IP assays. Shujie Xia, Jian Zhang, Joel B Nelson conceived study and helped with manuscript. Zhou Wang conceived and directed study, and helped with manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Jing, Y., Nguyen, M., Wang, D. et al. DHX15 promotes prostate cancer progression by stimulating Siah2-mediated ubiquitination of androgen receptor. Oncogene 37, 638–650 (2018). https://doi.org/10.1038/onc.2017.371
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.371
This article is cited by
-
Fusobacterium nucleatum promotes tumor progression in KRAS p.G12D-mutant colorectal cancer by binding to DHX15
Nature Communications (2024)
-
The DNA/RNA helicase DHX9 contributes to the transcriptional program of the androgen receptor in prostate cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
The RNA helicase DHX15 is a critical regulator of natural killer-cell homeostasis and functions
Cellular & Molecular Immunology (2022)
-
Functional roles of E3 ubiquitin ligases in prostate cancer
Journal of Molecular Medicine (2022)
-
DDX52 knockdown inhibits the growth of prostate cancer cells by regulating c-Myc signaling
Cancer Cell International (2021)