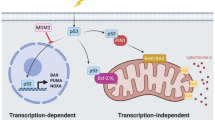
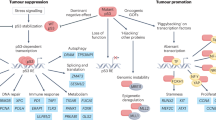
Abstract
pRb and p53 are the two major tumor suppressors. Their inactivation is frequent when cancers develop and their reactivation is rationale of most cancer therapeutics. When pRb and p53 are genetically inactivated, cells irreparably lose the antitumor mechanisms afforded by them. Cancer genome studies document recurrent genetic inactivation of RB1 and TP53, and the inactivation becomes more frequent in more advanced cancers. These findings may explain why more advanced cancers are more likely to resist current therapies. Finding successful treatments for more advanced and multi-therapy-resistant cancers will depend on finding antitumor mechanisms that remain effective when pRb and p53 are genetically inactivated. Here, we review studies that have begun to make progress in this direction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 2013; 152: 340–351.
Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 2014; 26: 136–149.
Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O et al. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell 2010; 17: 376–387.
Talluri S, Isaac CE, Ahmad M, Henley SA, Francis SM, Martens AL et al. A G1 checkpoint mediated by the retinoblastoma protein that is dispensable in terminal differentiation but essential for senescence. Mol Cell Biol 2010; 30: 948–960.
Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003; 113: 703–716.
Ianari A, Natale T, Calo E, Ferretti E, Alesse E, Screpanti I et al. Proapoptotic function of the retinoblastoma tumor suppressor protein. Cancer Cell 2009; 15: 184–194.
Dick FA, Dyson N . pRB contains an E2F1-specific binding domain that allows E2F1-induced apoptosis to be regulated separately from other E2F activities. Mol Cell 2003; 12: 639–649.
Shats I, Gatza ML, Liu B, Angus SP, You L, Nevins JR . FOXO transcription factors control E2F1 transcriptional specificity and apoptotic function. Cancer Res 2013; 73: 6056–6067.
Korenjak M, Anderssen E, Ramaswamy S, Whetstine JR, Dyson NJ . RBF binding to both canonical E2F targets and noncanonical targets depends on functional dE2F/dDP complexes. Mol Cell Biol 2012; 32: 4375–4387.
Nicolay BN, Gameiro PA, Tschop K, Korenjak M, Heilmann AM, Asara JM et al. Loss of RBF1 changes glutamine catabolism. Genes Dev 2013; 27: 182–196.
Hsieh MC, Das D, Sambandam N, Zhang MQ, Nahle Z . Regulation of the PDK4 isozyme by the Rb-E2F1 complex. J Biol Chem 2008; 283: 27410–27417.
Reynolds MR, Lane AN, Robertson B, Kemp S, Liu Y, Hill BG et al. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene 2014; 33: 556–566.
Clem BF, Chesney J . Molecular pathways: regulation of metabolism by RB. Clin Cancer Res 2012; 18: 6096–6100.
Nicolay BN, Dyson NJ . The multiple connections between pRB and cell metabolism. Curr Opin Cell Biol 2013; 25: 735–740.
Aksoy O, Chicas A, Zeng T, Zhao Z, McCurrach M, Wang X et al. The atypical E2F family member E2F7 couples the p53 and RB pathways during cellular senescence. Genes Dev 2012; 26: 1546–1557.
Carvajal LA, Hamard PJ, Tonnessen C, Manfredi JJ . E2F7, a novel target, is up-regulated by p53 and mediates DNA damage-dependent transcriptional repression. Genes Dev 2012; 26: 1533–1545.
Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI . The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 2006; 443: 214–217.
Efeyan A, Garcia-Cao I, Herranz D, Velasco-Miguel S, Serrano M . Tumour biology: policing of oncogene activity by p53. Nature 2006; 443: 159.
Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 2004; 5: 375–387.
Zindy F, Williams RT, Baudino TA, Rehg JE, Skapek SX, Cleveland JL et al. Arf tumor suppressor promoter monitors latent oncogenic signals in vivo. Proc Natl Acad Sci USA 2003; 100: 15930–15935.
Young NP, Jacks T . Tissue-specific p19Arf regulation dictates the response to oncogenic K-ras. Proc Natl Acad Sci USA 2010; 107: 10184–10189.
Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M et al. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell 2003; 4: 111–120.
Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M et al. Tumour biology: senescence in premalignant tumours. Nature 2005; 436: 642.
Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA . Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol 2007; 9: 493–505.
Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA et al. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell 2008; 14: 447–457.
Chen D, Kon N, Zhong J, Zhang P, Yu L, Gu W . Differential effects on ARF stability by normal versus oncogenic levels of c-Myc expression. Mol Cell 2013; 51: 46–56.
Junttila MR, Karnezis AN, Garcia D, Madriles F, Kortlever RM, Rostker F et al. Selective activation of p53-mediated tumour suppression in high-grade tumours. Nature 2010; 468: 567–571.
Feldser DM, Kostova KK, Winslow MM, Taylor SE, Cashman C, Whittaker CA et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature 2010; 468: 572–575.
Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L et al. Restoration of p53 function leads to tumour regression in vivo. Nature 2007; 445: 661–665.
Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007; 445: 656–660.
Martins CP, Brown-Swigart L, Evan GI . Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 2006; 127: 1323–1334.
Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 2012; 149: 1269–1283.
Valente LJ, Gray DH, Michalak EM, Pinon-Hofbauer J, Egle A, Scott CL et al. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell Reports 2013; 3: 1339–1345.
Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell 2011; 145: 571–583.
Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol 2011; 13: 317–323.
Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S et al. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med 2011; 208: 875–883.
Kim NH, Kim HS, Li XY, Lee I, Choi HS, Kang SE et al. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol 2011; 195: 417–433.
Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011; 17: 211–215.
Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J et al. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell 2008; 134: 62–73.
Yan HL, Xue G, Mei Q, Wang YZ, Ding FX, Liu MF et al. Repression of the miR-17-92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J 2009; 28: 2719–2732.
Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010; 142: 409–419.
Feng Z, Levine AJ . The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol 2010; 20: 427–434.
Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest 2010; 120: 681–693.
Astle MV, Hannan KM, Ng PY, Lee RS, George AJ, Hsu AK et al. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene 2012; 31: 1949–1962.
Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y et al. Regulation of PTEN transcription by p53. Mol Cell 2001; 8: 317–325.
Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S et al. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res 2007; 67: 3043–3053.
Budanov AV, Karin M . p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 2008; 134: 451–460.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014; 507: 315–322.
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010; 18: 11–22.
Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012; 487: 239–243.
Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068.
Xu XL, Fang Y, Lee TC, Forrest D, Gregory-Evans C, Almeida D et al. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell 2009; 137: 1018–1031.
Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C et al. Inactivation of the p53 pathway in retinoblastoma. Nature 2006; 444: 61–66.
Zhang J, Benavente CA, McEvoy J, Flores-Otero J, Ding L, Chen X et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 2012; 481: 329–334.
McEvoy J, Nagahawatte P, Finkelstein D, Richards-Yutz J, Valentine M, Ma J et al. RB1 gene inactivation by chromothripsis in human retinoblastoma. Oncotarget 2014; 5: 438–450.
Yamasaki L, Bronson R, Williams BO, Dyson NJ, Harlow E, Jacks T . Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1(+/-)mice. Nat Genet 1998; 18: 360–364.
Ziebold U, Lee EY, Bronson RT, Lees JA . E2F3 loss has opposing effects on different pRB-deficient tumors, resulting in suppression of pituitary tumors but metastasis of medullary thyroid carcinomas. Mol Cell Biol 2003; 23: 6542–6552.
Lee EY, Cam H, Ziebold U, Rayman JB, Lees JA, Dynlacht BD . E2F4 loss suppresses tumorigenesis in Rb mutant mice. Cancer Cell 2002; 2: 463–472.
Lasorella A, Rothschild G, Yokota Y, Russell RG, Iavarone A . Id2 mediates tumor initiation, proliferation, and angiogenesis in Rb mutant mice. Mol Cell Biol 2005; 25: 3563–3574.
Takahashi C, Contreras B, Bronson RT, Loda M, Ewen ME . Genetic interaction between Rb and K-ras in the control of differentiation and tumor suppression. Mol Cell Biol 2004; 24: 10406–10415.
Takahashi C, Contreras B, Iwanaga T, Takegami Y, Bakker A, Bronson RT et al. Nras loss induces metastatic conversion of Rb1-deficient neuroendocrine thyroid tumor. Nat Genet 2006; 38: 118–123.
Lin W, Cao J, Liu J, Beshiri ML, Fujiwara Y, Francis J et al. Loss of the retinoblastoma binding protein 2 (RBP2) histone demethylase suppresses tumorigenesis in mice lacking Rb1 or Men1. Proc Natl Acad Sci USA 2011; 108: 13379–13386.
Ji P, Jiang H, Rekhtman K, Bloom J, Ichetovkin M, Pagano M et al. An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol Cell 2004; 16: 47–58.
Binne UK, Classon MK, Dick FA, Wei W, Rape M, Kaelin WG Jr . et al. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat Cell Biol 2007; 9: 225–232.
Wang H, Bauzon F, Ji P, Xu X, Sun D, Locker J et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/- mice. Nat Genet 2010; 42: 83–88.
Malek NP, Sundberg H, McGrew S, Nakayama K, Kyriakidis TR, Roberts JM . A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature 2001; 413: 323–327.
Lu Z, Bauzon F, Fu H, Cui J, Zhao H, Nakayama K et al. Skp2 suppresses apoptosis in Rb1-deficient tumours by limiting E2F1 activity. Nat Commun 2014; 5: 3463.
Krek W, Ewen ME, Shirodkar S, Arany Z, Kaelin WG Jr ., Livingston DM . Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell 1994; 78: 161–172.
Krek W, Xu G, Livingston DM . Cyclin A-kinase regulation of E2F-1 DNA binding function underlies suppression of an S phase checkpoint. Cell 1995; 83: 1149–1158.
Dynlacht BD, Flores O, Lees JA, Harlow E . Differential regulation of E2F trans-activation by cyclin-cdk2 complexes. Genes Dev 1994; 8: 1772–1786.
Xu M, Sheppard KA, Peng CY, Yee AS, Piwnica-Worms H . Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol Cell Biol 1994; 14: 8420–8431.
Chen D, Livne-bar I, Vanderluit JL, Slack RS, Agochiya M, Bremner R . Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell 2004; 5: 539–551.
Zhang J, Schweers B, Dyer MA . The first knockout mouse model of retinoblastoma. Cell Cycle 2004; 3: 952–959.
Sangwan M, McCurdy SR, Livne-Bar I, Ahmad M, Wrana JL, Chen D et al. Established and new mouse models reveal E2f1 and Cdk2 dependency of retinoblastoma, and expose effective strategies to block tumor initiation. Oncogene 2012; 31: 5019–5028.
Besson A, Hwang HC, Cicero S, Donovan SL, Gurian-West M, Johnson D et al. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev 2007; 21: 1731–1746.
Zhu L, Harlow E, Dynlacht BD . p107 uses a p21CIP1-related domain to bind cyclin/cdk2 and regulate interactions with E2F. Genes Dev 1995; 9: 1740–1752.
Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005; 436: 725–730.
Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature 2010; 464: 374–379.
Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010; 463: 899–905.
Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol 2004; 6: 308–318.
Dang1 CV . MYC on the path to cancer. Cell 2012; 149: 22–35.
Soucek L, Whitfield J, Martins CP, Finch AJ, Murphy DJ, Sodir NM et al. Modelling Myc inhibition as a cancer therapy. Nature 2008; 455: 679–683.
Soucek L, Whitfield JR, Sodir NM, Masso-Valles D, Serrano E, Karnezis AN et al. Inhibition of Myc family proteins eradicates KRas-driven lung cancer in mice. Genes Dev 2013; 27: 504–513.
Wu CH, van Riggelen J, Yetil A, Fan AC, Bachireddy P, Felsher DW . Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci USA 2007; 104: 13028–13033.
Sodir NM, Swigart LB, Karnezis AN, Hanahan D, Evan GI, Soucek L . Endogenous Myc maintains the tumor microenvironment. Genes Dev 2011; 25: 907–916.
Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011; 146: 904–917.
Haley VL, Barnes DJ, Sandovici I, Constancia M, Graham CF, Pezzella F et al. Igf2 pathway dependency of the Trp53 developmental and tumour phenotypes. EMBO Mol Med 2012; 4: 705–718.
Clermont F, Nittner D, Marine JC . IGF2: the Achilles' heel of p53-deficiency? EMBO Mol Med 2012; 4: 688–690.
Emerling BM, Hurov JB, Poulogiannis G, Tsukazawa KS, Choo-Wing R, Wulf GM et al. Depletion of a putatively druggable class of phosphatidylinositol kinases inhibits growth of p53-null tumors Cell 2013; 155: 844–857.
Reinhardt HC, Jiang H, Hemann MT, Yaffe MB . Exploiting synthetic lethal interactions for targeted cancer therapy. Cell Cycle 2009; 8: 3112–3119.
Manke IA, Nguyen A, Lim D, Stewart MQ, Elia AE, Yaffe MB . MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol Cell 2005; 17: 37–48.
Reinhardt HC, Hasskamp P, Schmedding I, Morandell S, van Vugt MA, Wang X et al. DNA damage activates a spatially distinct late cytoplasmic cell-cycle checkpoint network controlled by MK2-mediated RNA stabilization. Mol Cell 2010; 40: 34–49.
Cannell IG, Kong YW, Johnston SJ, Chen ML, Collins HM, Dobbyn HC et al. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. Proc Natl Acad Sci USA 2010; 107: 5375–5380.
Kopper F, Bierwirth C, Schon M, Kunze M, Elvers I, Kranz D et al. Damage-induced DNA replication stalling relies on MAPK-activated protein kinase 2 activity. Proc Natl Acad Sci USA 2013; 110: 16856–16861.
Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB . p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell 2007; 11: 175–189.
Jiang H, Reinhardt HC, Bartkova J, Tommiska J, Blomqvist C, Nevanlinna H et al. The combined status of ATM and p53 link tumor development with therapeutic response. Genes Dev 2009; 23: 1895–1909.
Morandell S, Reinhardt HC, Cannell IG, Kim JS, Ruf DM, Mitra T et al. A reversible gene-targeting strategy identifies synthetic lethal interactions between MK2 and p53 in the DNA damage response in vivo. Cell Reports 2013; 5: 868–877.
Berkers CR, Maddocks OD, Cheung EC, Mor I, Vousden KH . Metabolic regulation by p53 family members. Cell Metab 2013; 18: 617–633.
Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 2006; 126: 107–120.
Lee P, Vousden KH, Cheung EC . TIGAR TIGAR, burning bright. Cancer Metab 2014; 2: 1.
Cheung EC, Athineos D, Lee P, Ridgway RA, Lambie W, Nixon C et al. TIGAR is required for efficient intestinal regeneration and tumorigenesis. Dev Cell 2013; 25: 463–477.
Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z . Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci USA 2010; 107: 7455–7460.
Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci USA 2010; 107: 7461–7466.
Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol 2011; 13: 310–316.
Jiang P, Du W, Mancuso A, Wellen KE, Yang X . Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 2013; 493: 689–693.
Maddocks OD, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 2013; 493: 542–546.
Zhao H, Bauzon F, Fu H, Lu Z, Cui J, Nakayama K et al. Skp2 deletion unmasks a p27 safeguard that blocks tumorigenesis in the absence of pRb and p53 tumor suppressors. Cancer Cell 2013; 24: 645–659.
Starostina NG, Kipreos ET . Multiple degradation pathways regulate versatile CIP/KIP CDK inhibitors. Trends Cell Biol 2012; 22: 33–41.
Mogilyansky E, Rigoutsos I . The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ 2013; 20: 1603–1614.
O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT . c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005; 435: 839–843.
He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S et al. A microRNA polycistron as a potential human oncogene. Nature 2005; 435: 828–833.
Sears R, Ohtani K, Nevins JR . Identification of positively and negatively acting elements regulating expression of the E2F2 gene in response to cell growth signals. Mol Cell Biol 1997; 17: 5227–5235.
Adams MR, Sears R, Nuckolls F, Leone G, Nevins JR . Complex transcriptional regulatory mechanisms control expression of the E2F3 locus. Mol Cell Biol 2000; 20: 3633–3639.
Hiebert SW, Lipp M, Nevins JR . E1A-dependent trans-activation of the human MYC promoter is mediated by the E2F factor. Proc Natl Acad Sci USA 1989; 86: 3594–3598.
Sylvestre Y, De Guire V, Querido E, Mukhopadhyay UK, Bourdeau V, Major F et al. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem 2007; 282: 2135–2143.
Woods K, Thomson JM, Hammond SM . Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors. J Biol Chem 2007; 282: 2130–2134.
Conkrite K, Sundby M, Mukai S, Thomson JM, Mu D, Hammond SM et al. miR-17~92 cooperates with RB pathway mutations to promote retinoblastoma. Genes Dev 2011; 25: 1734–1745.
Nittner D, Lambertz I, Clermont F, Mestdagh P, Kohler C, Nielsen SJ et al. Synthetic lethality between Rb, p53 and Dicer or miR-17-92 in retinal progenitors suppresses retinoblastoma formation. Nat Cell Biol 2012; 14: 958–965.
Li B, Gordon GM, Du CH, Xu J, Du W . Specific killing of Rb mutant cancer cells by inactivating TSC2. Cancer Cell 2010; 17: 469–480.
Zhang T, Liao Y, Hsu FN, Zhang R, Searle JS, Pei X et al. Hyperactivated Wnt signaling induces synthetic lethal interaction with Rb inactivation by elevating TORC1 activities. PLoS Genet 2014; 10: e1004357.
Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell 2012; 149: 1098–1111.
Inuzuka H, Gao D, Finley LW, Yang W, Wan L, Fukushima H et al. Acetylation-dependent regulation of Skp2 function. Cell 2012; 150: 179–193.
Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008; 132: 875–886.
Chen Q, Xie W, Kuhn DJ, Voorhees PM, Lopez-Girona A, Mendy D et al. Targeting the p27 E3 ligase SCF(Skp2) results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood 2008; 111: 4690–4699.
Rico-Bautista E, Yang CC, Lu L, Roth GP, Wolf DA . Chemical genetics approach to restoring p27Kip1 reveals novel compounds with antiproliferative activity in prostate cancer cells. BMC Biol 2010; 8: 153.
Chan CH, Morrow JK, Li CF, Gao Y, Jin G, Moten A et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression Cell 2013; 154: 556–568.
Spruck C, Strohmaier H, Watson M, Smith APL, Ryan A, Krek W et al. A CDK-independent function of mammalian Cks1: targeting of SCFSkp2 to the CDK inhibitor p27Kip1. Mol Cell 2001; 7: 639–650.
Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M et al. The cell-cycle regulatory protein Cks1 is required for SCFSkp2-mediated ubiquitinylation of p27. Nat Cell Biol 2001; 3: 321–324.
Wu L, Grigoryan AV, Li Y, Hao B, Pagano M, Cardozo TJ . Specific small molecule inhibitors of Skp2-mediated p27 degradation. Chem Biol 2012; 19: 1515–1524.
Ooi LC, Watanabe N, Futamura Y, Sulaiman SF, Darah I, Osada H . Identification of small molecule inhibitors of p27(Kip1) ubiquitination by high-throughput screening. Cancer Sci 2013; 104: 1461–1467.
Ungermannova D, Lee J, Zhang G, Dallmann HG, McHenry CS, Liu X . High-throughput screening AlphaScreen assay for identification of small-molecule inhibitors of ubiquitin E3 ligase SCFSkp2-Cks1. J Biomol Screen 2013; 18: 910–920.
Nickeleit I, Zender S, Sasse F, Geffers R, Brandes G, Sorensen I et al. Argyrin a reveals a critical role for the tumor suppressor protein p27(kip1) in mediating antitumor activities in response to proteasome inhibition. Cancer Cell 2008; 14: 23–35.
Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C et al. Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet 2011; 43: 371–378.
van Rooij E, Kauppinen S . Development of microRNA therapeutics is coming of age. EMBO Mol Med 2014; 6: 851–864.
Murphy BL, Obad S, Bihannic L, Ayrault O, Zindy F, Kauppinen S et al. Silencing of the miR-17~92 cluster family inhibits medulloblastoma progression. Cancer Res 2013; 73: 7068–7078.
Muller PA, Trinidad AG, Caswell PT, Norman JC, Vousden KH . Mutant p53 regulates Dicer through p63-dependent and -independent mechanisms to promote an invasive phenotype. J Biol Chem 2014; 289: 122–132.
Freed-Pastor WA, Mizuno H, Zhao X, Langerod A, Moon SH, Rodriguez-Barrueco R et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 2012; 148: 244–258.
Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005; 7: 469–483.
Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci USA 2010; 107: 246–251.
Weissmueller S, Manchado E, Saborowski M, Morris JPt, Wagenblast E, Davis CA et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell 2014; 157: 382–394.
Jackson JG, Lozano G . The mutant p53 mouse as a pre-clinical model. Oncogene 2013; 32: 4325–4330.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Burkhart DL, Sage J . Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer 2008; 8: 671–682.
Dick FA, Rubin SM . Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol 2013; 14: 297–306.
Kruse JP, Gu W . Modes of p53 regulation. Cell 2009; 137: 609–622.
Bieging KT, Mello SS, Attardi LD . Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer 2014; 14: 359–370.
Dang CV . Links between metabolism and cancer. Genes Dev 2012; 26: 877–890.
De Craene B, Berx G . Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013; 13: 97–110.
Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA 2005; 102: 1649–1654.
Acknowledgements
Work in the authors’ laboratory was supported by NIH grants RO1CA127901 and RO1CA131421. HZ is a recipient of DOD PCRP Postdoctoral Fellowship (PC121837), and LZ acknowledges support from the Irma T Hirschl Career Scientist Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhu, L., Lu, Z. & Zhao, H. Antitumor mechanisms when pRb and p53 are genetically inactivated. Oncogene 34, 4547–4557 (2015). https://doi.org/10.1038/onc.2014.399
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.399
This article is cited by
-
Identification and validation of an E2F-related gene signature for predicting recurrence-free survival in human prostate cancer
Cancer Cell International (2022)
-
Targeting the untargetable: RB1-deficient tumours are vulnerable to Skp2 ubiquitin ligase inhibition
British Journal of Cancer (2022)
-
Effects of concomitant inactivation of p53 and pRb on response to doxorubicin treatment in breast cancer cell lines
Cell Death Discovery (2017)
-
The p53 tetramer shows an induced-fit interaction of the C-terminal domain with the DNA-binding domain
Oncogene (2016)
-
Kv10.1 K+ channel: from physiology to cancer
Pflügers Archiv - European Journal of Physiology (2016)