Abstract
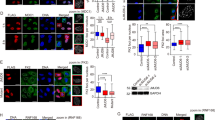
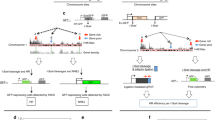
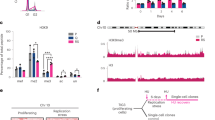
Constitutive heterochromatin (HC) is important for maintaining chromosome stability, but also delays the repair of DNA double-strand breaks (DSBs). DSB repair in complex mammalian genomes involves a fast phase (2–6 h) in which most of the breaks are rapidly repaired, and a slow phase (up to 24 h) in which the remaining damages in HC are repaired. We found that p53 deficiency delays the slow-phase DNA repair after ionizing irradiation. p53 deficiency prevents downregulation of histone H3K9 trimethylation at the pericentric HC after DNA damage. Moreover, p53 directly induces expression of the H3K9 demethylase Jumonji domain 2 family demethylase (JMJD2b) through promoter binding. The p53 activation also indirectly downregulates expression of the H3K9 methyltransferase SUV39H1. Depletion of JMJD2b or sustained expression of SUV39H1 delays the repair of HC DNA and reduces clonogenic survival after ionizing irradiation. The results suggest that by regulating JMJD2b and SUV39H1 expression, p53 not only controls transcription but also promotes HC relaxation to accelerate a rate-limiting step in the repair of complex genomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Kastan MB, Lim DS . The many substrates and functions of ATM. Nat Rev Mol Cell Biol 2000; 1: 179–186.
Kemp CJ, Wheldon T, Balmain A . p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet 1994; 8: 66–69.
Donehower LA, Godley LA, Aldaz CM, Pyle R, Shi YP, Pinkel D et al. Deficiency of p53 accelerates mammary tumorigenesis in Wnt-1 transgenic mice and promotes chromosomal instability. Genes Dev 1995; 9: 882–895.
Harvey M, Sands AT, Weiss RS, Hegi ME, Wiseman RW, Pantazis P et al. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene 1993; 8: 2457–2467.
Bischoff FZ, Yim SO, Pathak S, Grant G, Siciliano MJ, Giovanella BC et al. Spontaneous abnormalities in normal fibroblasts from patients with Li-Fraumeni cancer syndrome: aneuploidy and immortalization. Cancer Res 1990; 50: 7979–7984.
Bouffler SD, Kemp CJ, Balmain A, Cox R . Spontaneous and ionizing radiation-induced chromosomal abnormalities in p53-deficient mice. Cancer Res 1995; 55: 3883–3889.
Bishop AJ, Hollander MC, Kosaras B, Sidman RL, Fornace AJ, Schiestl RH . Atm-, p53-, and Gadd45a-deficient mice show an increased frequency of homologous recombination at different stages during development. Cancer Res 2003; 63: 5335–5343.
Mekeel KL, Tang W, Kachnic LA, Luo CM, DeFrank JS, Powell SN . Inactivation of p53 results in high rates of homologous recombination. Oncogene 1997; 14: 1847–1857.
Helton ES, Chen X . p53 modulation of the DNA damage response. J Cell Biochem 2007; 100: 883–896.
Sengupta S, Harris CC . p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol 2005; 6: 44–55.
Yan W, Chen X . Characterization of functional domains necessary for mutant p53 gain of function. J Biol Chem 2010; 285: 14229–14238.
Cuddihy AR, Bristow RG . The p53 protein family and radiation sensitivity: Yes or no? Cancer Metastasis Rev 2004; 23: 237–257.
Gudkov AV, Komarova EA . The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 2003; 3: 117–129.
Kuhne M, Riballo E, Rief N, Rothkamm K, Jeggo PA, Lobrich M . A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res 2004; 64: 500–508.
Goodarzi AA, Jeggo P, Lobrich M . The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax. DNA Repair (Amst) 2010; 9: 1273–1282.
Puerto S, Ramirez MJ, Marcos R, Creus A, Surralles J . Radiation-induced chromosome aberrations in human euchromatic (17cen-p53) and heterochromatic (1cen-1q12) regions. Mutagenesis 2001; 16: 291–296.
Cowell IG, Sunter NJ, Singh PB, Austin CA, Durkacz BW, Tilby MJ . gammaH2AX foci form preferentially in euchromatin after ionising-radiation. PloS one 2007; 2: e1057.
Murga M, Jaco I, Fan Y, Soria R, Martinez-Pastor B, Cuadrado M et al. Global chromatin compaction limits the strength of the DNA damage response. J Cell Biol 2007; 178: 1101–1108.
Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell 2008; 31: 167–177.
Ziv Y, Bielopolski D, Galanty Y, Lukas C, Taya Y, Schultz DC et al. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat Cell Biol 2006; 8: 870–876.
Chiolo I, Minoda A, Colmenares SU, Polyzos A, Costes SV, Karpen GH . Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 2011; 144: 732–744.
Peters AH, O'Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 2001; 107: 323–337.
Martens JH, O'Sullivan RJ, Braunschweig U, Opravil S, Radolf M, Steinlein P et al. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J 2005; 24: 800–812.
Garcia-Cao M, O'Sullivan R, Peters AH, Jenuwein T, Blasco MA . Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat Genet 2004; 36: 94–99.
Peng JC, Karpen GH . Heterochromatic genome stability requires regulators of histone H3 K9 methylation. Plos Genet 2009; 5: e1000435.
Fodor BD, Kubicek S, Yonezawa M, O'Sullivan RJ, Sengupta R, Perez-Burgos L et al. Jmjd2b antagonizes H3K9 trimethylation at pericentric heterochromatin in mammalian cells. Genes Dev 2006; 20: 1557–1562.
Slee RB, Steiner CM, Herbert BS, Vance GH, Hickey RJ, Schwarz T et al. Cancer-associated alteration of pericentromeric heterochromatin may contribute to chromosome instability. Oncogene 2011; 31: 3244–3253.
Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest 1999; 104: 263–269.
Cheng Q, Chen L, Li Z, Lane WS, Chen J . ATM activates p53 by regulating MDM2 oligomerization and E3 processivity. EMBO J 2009; 28: 3857–3867.
Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S et al. GammaH2AX and cancer. Nat Rev Cancer 2008; 8: 957–967.
Finelli P, Antonacci R, Marzella R, Lonoce A, Archidiacono N, Rocchi M . Structural organization of multiple alphoid subsets coexisting on human chromosomes 1, 4, 5, 7, 9, 15, 18, and 19. Genomics 1996; 38: 325–330.
Chen L, Li Z, Zwolinska AK, Smith MA, Cross B, Koomen J et al. MDM2 recruitment of lysine methyltransferases regulates p53 transcriptional output. EMBO J 2010; 29: 2538–2552.
Bosch-Presegue L, Raurell-Vila H, Marazuela-Duque A, Kane-Goldsmith N, Valle A, Oliver J et al. Stabilization of Suv39H1 by SirT1 is part of oxidative stress response and ensures genome protection. Mol Cell 2011; 42: 210–223.
Tjeertes JV, Miller KM, Jackson SP . Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J 2009; 28: 1878–1889.
Goodier JL, Kazazian HH . Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell 2008; 135: 23–35.
Kondo Y, Issa JP . Enrichment for histone H3 lysine 9 methylation at Alu repeats in human cells. J Biol Chem 2003; 278: 27658–27662.
el-Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B . Definition of a consensus binding site for p53. Nat Genet 1992; 1: 45–49.
Lohr K, Moritz C, Contente A, Dobbelstein M . p21/CDKN1A mediates negative regulation of transcription by p53. J Biol Chem 2003; 278: 32507–32516.
Takahashi K, Kaneko I . Changes in nuclease sensitivity of mammalian cells after irradiation with 60Co gamma-rays. Int J Radiat Biol Relat Stud Phys Chem Med 1985; 48: 389–395.
Carrier F, Georgel PT, Pourquier P, Blake M, Kontny HU, Antinore MJ et al. Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol Cell Biol 1999; 19: 1673–1685.
Rubbi CP, Milner J . p53 is a chromatin accessibility factor for nucleotide excision repair of DNA damage. EMBO J 2003; 22: 975–986.
Mungamuri SK, Benson EK, Wang S, Gu W, Lee SW, Aaronson SA . p53-mediated heterochromatin reorganization regulates its cell fate decisions. Nat Struct Mol Biol 2012; 19: 478–484.
Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 2007; 448: 553–560.
Ohki S, Onda M, Fukushima T, Takita K, Suzuki S, Hoshino M et al. Telomeric repeat-length alterations in colorectal carcinoma are associated with loss of heterozygosity and point mutation in p53 gene. Int J Mol Med 2003; 11: 485–490.
Hiyama K, Ishioka S, Shirotani Y, Inai K, Hiyama E, Murakami I et al. Alterations in telomeric repeat length in lung cancer are associated with loss of heterozygosity in p53 and Rb. Oncogene 1995; 10: 937–944.
Dikomey E, Dahm-Daphi J, Brammer I, Martensen R, Kaina B . Correlation between cellular radiosensitivity and non-repaired double-strand breaks studied in nine mammalian cell lines. Int J Radiat Biol 1998; 73: 269–278.
Taneja N, Davis M, Choy JS, Beckett MA, Singh R, Kron SJ et al. Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J Biol Chem 2004; 279: 2273–2280.
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J et al. Initial sequencing and analysis of the human genome. Nature 2001; 409: 860–921.
Scott SL, Earle JD, Gumerlock PH . Functional p53 increases prostate cancer cell survival after exposure to fractionated doses of ionizing radiation. Cancer Res 2003; 63: 7190–7196.
Banath JP, Macphail SH, Olive PL . Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res 2004; 64: 7144–7149.
Bertheau P, Turpin E, Rickman DS, Espie M, de Reynies A, Feugeas JP et al. Exquisite sensitivity of TP53 mutant and basal breast cancers to a dose-dense epirubicin-cyclophosphamide regimen. PLOS Med 2007; 4: e90.
Bertheau P, Plassa F, Espie M, Turpin E, de Roquancourt A, Marty M et al. Effect of mutated TP53 on response of advanced breast cancers to high-dose chemotherapy. Lancet 2002; 360: 852–854.
Kroger N, Milde-Langosch K, Riethdorf S, Schmoor C, Schumacher M, Zander AR et al. Prognostic and predictive effects of immunohistochemical factors in high-risk primary breast cancer patients. Clin Cancer Res 2006; 12: 159–168.
Jackson JG, Pant V, Li Q, Chang LL, Quintas-Cardama A, Garza D et al. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell 2012; 21: 793–806.
Palomera-Sanchez Z, Bucio-Mendez A, Valadez-Graham V, Reynaud E, Zurita M . Drosophila p53 is required to increase the levels of the dKDM4B demethylase after UV-induced DNA damage to demethylate histone H3 lysine 9. J Biol Chem 2010; 285: 31370–31379.
Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 2003; 22: 4212–4222.
Huang B, Deo D, Xia M, Vassilev LT . Pharmacologic p53 activation blocks cell cycle progression but fails to induce senescence in epithelial cancer cells. Mol Cancer Res 2009; 7: 1497–1509.
Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003; 113: 703–716.
Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 2005; 436: 660–665.
Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR . HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature 2008; 453: 682–686.
Ayoub N, Jeyasekharan AD, Venkitaraman AR . Mobilization and recruitment of HP1: a bimodal response to DNA breakage. Cell Cycle 2009; 8: 2945–2950.
Luijsterburg MS, Dinant C, Lans H, Stap J, Wiernasz E, Lagerwerf S et al. Heterochromatin protein 1 is recruited to various types of DNA damage. J Cell Biol 2009; 185: 577–586.
Sharma SS, Ma L, Bagui TK, Forinash KD, Pledger WJA . p27(Kip1) mutant that does not inhibit CDK activity promotes centrosome amplification and micronucleation. Oncogene 2011; 31: 3989–3998.
Boyd KE, Wells J, Gutman J, Bartley SM, Farnham PJ . c-Myc target gene specificity is determined by a post-DNAbinding mechanism. Proc Natl Acad Sci USA 1998; 95: 13887–13892.
Batzer MA, Alegria-Hartman M, Deininger PL . A consensus Alu repeat probe for physical mapping. Genet Anal Tech Appl 1994; 11: 34–38.
Acknowledgements
We thank the Moffitt Molecular Genomics Core for DNA sequence analyses, and Analytic Microscopy Core for image quantification. We also want to thank Dr. Savitha Sharma for providing the inducible p27 cell line. H Zheng is supported by a postdoctoral fellowship from the Florida Department of Health James and Esther King Biomedical Research Program. This work is supported by grants from the National Institutes of Health to J Chen (CA141244, CA109636).
Author contribution: Hong Zheng and Lihong Chen designed and performed the experiments. W Jack Pledger and Jia Fang provided important intellectual input and reagents. Hong Zheng and Jiandong Chen designed the experiments and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zheng, H., Chen, L., Pledger, W. et al. p53 promotes repair of heterochromatin DNA by regulating JMJD2b and SUV39H1 expression. Oncogene 33, 734–744 (2014). https://doi.org/10.1038/onc.2013.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.6
Keywords
This article is cited by
-
The emerging roles of histone demethylases in cancers
Cancer and Metastasis Reviews (2024)
-
Human endogenous retroviruses as epigenetic therapeutic targets in TP53-mutated diffuse large B-cell lymphoma
Signal Transduction and Targeted Therapy (2023)
-
Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity
Oncogene (2021)
-
The chemotherapeutic drug carboplatin affects macrophage responses to LPS and LPS tolerance via epigenetic modifications
Scientific Reports (2021)
-
JMJD2 promotes acquired cisplatin resistance in non-small cell lung carcinoma cells
Oncogene (2019)