Abstract
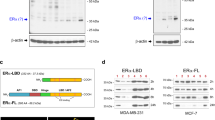
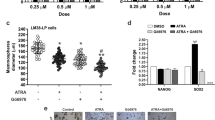
Tamoxifen (Tam) treatment is a first-line endocrine therapy for estrogen receptor-α-positive breast cancer patients. Unfortunately, resistance frequently occurs and is often related with overexpression of the membrane tyrosine kinase receptor HER2. This is the rationale behind combined treatments with endocrine therapy and novel inhibitors that reduce HER2 expression and signaling and thus inhibit Tam-resistant breast cancer cell growth. In this study, we show that activation of farnesoid X receptor (FXR), by the primary bile acid chenodeoxycholic acid (CDCA) or the synthetic agonist GW4064, inhibited growth of Tam-resistant breast cancer cells (termed MCF-7 TR1), which was used as an in vitro model of acquired Tam resistance. Our results demonstrate that CDCA treatment significantly reduced both anchorage-dependent and anchorage-independent epidermal growth factor (EGF)-induced growth in MCF-7 TR1 cells. Furthermore, results from western blot analysis and real-time reverse transcription–PCR revealed that CDCA treatment reduced HER2 expression and inhibited EGF-mediated HER2 and p42/44 mitogen-activated protein kinase (MAPK) phosphorylation in these Tam-resistant breast cancer cells. Transient transfection experiments, using a vector containing the human HER2 promoter region, showed that CDCA treatment downregulated basal HER2 promoter activity. This occurred through an inhibition of nuclear factor-κB transcription factor binding to its specific responsive element located in the HER2 promoter region as revealed by mutagenesis studies, electrophoretic mobility shift assay and chromatin immunoprecipitation analysis. Collectively, these data suggest that FXR ligand-dependent activity, blocking HER2/MAPK signaling, may overcome anti-estrogen resistance in human breast cancer cells and could represent a new therapeutic tool to treat breast cancer patients that develop resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Allred DC, Clark GM, Molina R, Tandon AK, Schnitt SJ, Gilchrist KW et al. (1992). Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol 23: 974–979.
Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ . (2001). Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem 276: 28857–28865.
Andrews NC, Faller DV . (1991). A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res 19: 2499.
Arpino G, Green SJ, Allred DC, Lew D, Martino S, Osborne CK et al. (2004). HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin Cancer Res 10: 5670–5676.
Arpino G, Wiechmann L, Osborne CK, Schiff R . (2008). Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev 29: 217–233.
Barone I, Brusco L, Gu G, Selever J, Beyer A, Covington KR et al. (2011). Loss of Rho GDI α and resistance to tamoxifen via effects on estrogen receptor α. Journal Natl Cancer Inst (in press).
Barone I, Cui Y, Herynk MH, Corona-Rodriguez A, Giordano C, Selever J et al. (2009). Expression of the K303R estrogen receptor-alpha breast cancer mutation induces resistance to an aromatase inhibitor via addiction to the PI3K/Akt kinase pathway. Cancer Res 69: 4724–4732.
Bishop-Bailey D . (2004). FXR as a novel therapeutic target for vascular disease. Drug News Perspect 17: 499–504.
Blume-Jensen P, Hunter T . (2001). Oncogenic kinase signalling. Nature 411: 355–365.
Catalano S, Malivindi R, Giordano C, Gu G, Panza S, Bonofiglio D et al. (2010). Farnesoid X receptor, through the binding with steroidogenic factor 1-responsive element, inhibits aromatase expression in tumor Leydig cells. J Biol Chem 285: 5581–5593.
Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH . (2002). Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer 97: 306–312.
Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V et al. (2002). Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J Clin Invest 109: 961–971.
De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F et al. (2005). A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res 11: 4741–4748.
Encarnacion CA, Ciocca DR, McGuire WL, Clark GM, Fuqua SA, Osborne CK . (1993). Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res Treat 26: 237–246.
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM et al. (1998). Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90: 1371–1388.
Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T et al. (1995). Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81: 687–693.
Giordano C, Cui Y, Barone I, Andò S, Mancini MA, Berno V et al. (2010). Growth factor-induced resistance to tamoxifen is associated with a mutation of estrogen receptor alpha and its phosphorylation at serine 305. Breast Cancer Res Treat 119: 71–85.
Glockner S, Lehmann U, Wilke N, Kleeberger W, Langer F, Kreipe H . (2001). Amplification of growth regulatory genes in intraductal breast cancer is associated with higher nuclear grade but not with the progression to invasiveness. Lab Invest 81: 565–571.
Gradishar WJ . (2004). Tamoxifen—what next? Oncologist 9: 378–384.
Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC et al. (2005). Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol 23: 2469–2476.
He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A et al. (2006). Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res 98: 192–199.
Herynk MH, Beyer AR, Cui Y, Weiss H, Anderson E, Green TP et al. (2006). Cooperative action of tamoxifen and c-Src inhibition in preventing the growth of estrogen receptor-positive human breast cancer cells. Mol Cancer Ther 5: 3023–3031.
Hurst HC . (2001). Update on HER-2 as a target for cancer therapy: the ERBB2 promoter and its exploitation for cancer treatment. Breast Cancer Res 3: 395–398.
Ishii S, Imamoto F, Yamanashi Y, Toyoshima K, Yamamoto T . (1987). Characterization of the promoter region of the human c-erbB-2 protooncogene. Proc Natl Acad Sci USA 84: 4374–4378.
Johnston SR . (2009). Enhancing the efficacy of hormonal agents with selected targeted agents. Clin Breast Cancer 9 (Suppl 1): S28–S36.
Journe F, Laurent G, Chaboteaux C, Nonclercq D, Durbecq V, Larsimont D et al. (2008). Farnesol, a mevalonate pathway intermediate, stimulates MCF-7 breast cancer cell growth through farnesoid-X-receptor-mediated estrogen receptor activation. Breast Cancer Res Treat 107: 49–61.
Kalaany NY, Mangelsdorf DJ . (2006). LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol 68: 159–191.
Kirkegaard T, McGlynn LM, Campbell FM, Muller S, Tovey SM, Dunne B et al. (2007). Amplified in breast cancer 1 in human epidermal growth factor receptor—positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res 13: 1405–1411.
Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME et al. (2003). Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 144: 1032–1044.
Kocarek TA, Shenoy SD, Mercer-Haines NA, Runge-Morris M . (2002). Use of dominant negative nuclear receptors to study xenobiotic-inducible gene expression in primary cultured hepatocytes. J Pharmacol Toxicol Methods 47: 177–187.
Laffitte BA, Kast HR, Nguyen CM, Zavacki AM, Moore DD, Edwards PA . (2000). Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem 275: 10638–10647.
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A et al. (1999). Identification of a nuclear receptor for bile acids. Science 284: 1362–1365.
Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S et al. (2004). HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA 101: 9393–9398.
Morelli C, Garofalo C, Sisci D, del Rincon S, Cascio S, Tu X et al. (2004). Nuclear insulin receptor substrate 1 interacts with estrogen receptor alpha at ERE promoters. Oncogene 23: 7517–7526.
Nicholson RI, Staka C, Boyns F, Hutcheson IR, Gee JM . (2004). Growth factor-driven mechanisms associated with resistance to estrogen deprivation in breast cancer: new opportunities for therapy. Endocr Relat Cancer 11: 623–641.
Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA et al. (2003). Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95: 353–361.
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA et al. (1999). Bile acids: natural ligands for an orphan nuclear receptor. Science 284: 1365–1368.
Sabnis GJ, Jelovac D, Long B, Brodie A . (2005). The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res 65: 3903–3910.
Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK . (2004). Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res 10: 331S–336S.
Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A et al. (2001). Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol 19: 2587–2595.
Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H et al. (2004). Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96: 926–935.
Sirianni R, Chimento A, Malivindi R, Mazzitelli I, Ando S, Pezzi V . (2007). Insulin-like growth factor-I, regulating aromatase expression through steroidogenic factor 1, supports estrogen-dependent tumor Leydig cell proliferation. Cancer Res 67: 8368–8377.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A et al. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792.
Staka CM, Nicholson RI, Gee JM . (2005). Acquired resistance to oestrogen deprivation: role for growth factor signalling kinases/oestrogen receptor cross-talk revealed in new MCF-7X model. Endocr Relat Cancer 12 (Suppl 1): S85–S97.
Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, Bishop-Bailey D . (2006). The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res 66: 10120–10126.
Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S . (2009). The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol 183: 6251–6261.
Xing X, Wang SC, Xia W, Zou Y, Shao R, Kwong KY et al. (2000). The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat Med 6: 189–195.
Yarden Y . (2001). Biology of HER2 and its importance in breast cancer. Oncology 61 (Suppl 2): 1–13.
Acknowledgements
This work was supported by AIRC grants, MIUR Ex 60% 2009, PRIN 2009 and Lilli Funaro Foundation. NIH/NCI R01 CA72038 and RP101251 from the Cancer Prevention and Research Institute of Texas (SAWF). We thank Dr Mien-Chie Hung for providing the pNeuLite plasmid and Dr T.A. Kocarek for providing the FXR-responsive reporter gene and FXR-DN expression plasmids. We also thank Dr Rachel Schiff for providing the MCF-7/HER2-18 cells.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Giordano, C., Catalano, S., Panza, S. et al. Farnesoid X receptor inhibits tamoxifen-resistant MCF-7 breast cancer cell growth through downregulation of HER2 expression. Oncogene 30, 4129–4140 (2011). https://doi.org/10.1038/onc.2011.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.124
Keywords
This article is cited by
-
The role of bile acids in carcinogenesis
Cellular and Molecular Life Sciences (2022)
-
Cytotoxicity of synthetic derivatives against breast cancer and multi-drug resistant breast cancer cell lines: a literature-based perspective study
Cancer Cell International (2021)
-
Targeting Farnesoid X receptor (FXR) for developing novel therapeutics against cancer
Molecular Biomedicine (2021)
-
Expression signatures and roles of microRNAs in inflammatory breast cancer
Cancer Cell International (2019)
-
Activated FXR Inhibits Leptin Signaling and Counteracts Tumor-promoting Activities of Cancer-Associated Fibroblasts in Breast Malignancy
Scientific Reports (2016)