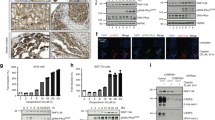
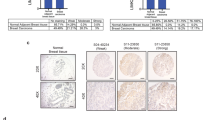
Abstract
Hsp90 chaperones stabilize many tyrosine kinases including several oncogenes, which are inhibited or induced to degrade by the Hsp90 inhibitor geldanamycin (GA). As a consequence, GA has been developed for future chemotherapeutic use in several tumour types including neuroblastoma (NB). Alternative splicing of the neurotrophin receptor tyrosine kinase TrkA may have a pivotal function in regulating NB behaviour, with reports suggesting that tumour-suppressing signals from TrkA may be converted to oncogenic signals by stress-regulated alternative TrkAIII splicing. Within this context, it is important to know whether Hsp90 interacts with TrkA variants in NB cells and how GA influences this. Here, we report that both TrkAI and TrkAIII are Hsp90 clients in human NB cells. TrkAI exhibits GA-sensitive interaction with Hsp90 required for receptor endoplasmic reticulum export, maturation, cell surface stabilization and ligand-mediated activation, whereas TrkAIII exhibits GA-sensitive interactions with Hsp90 required for spontaneous activity and to a lesser extent stability. We show that GA inhibits proliferation and induces apoptosis of TrkAI expressing NB cells, whereas TrkAIII reduces the sensitivity of NB cells to GA-induced elimination. Our data suggest that GA-sensitive interactions with Hsp90 are critical for both TrkAI tumour suppressor and TrkAIII oncogenic function in NB and that TrkAIII expression exerts a negative impact on GA-induced NB cell eradication, which can be counteracted by a novel TrkAIII-specific peptide nucleic acid inhibitor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Arevalo JC, Conde B, Hempstead BL, Chao MV, Martin-Zanca D, Perez P . (2000). TrkA immunoglobulin-like ligand binding domains inhibit spontaneous activation of the receptor. Mol Cell Biol 20: 5908–5916.
Bagatell R, Gore L, Egorin MJ, Ho R, Heller G, Boucher N et al. (2007). Phase I pharmacokinetic and pharmacodynamic study of 17-N-allylamino-17-demethoxygeldanamycin in paediatric patients with recurrent or refractory solid tumors: a pediatric oncology experimental therapeutics investigators corsortium study. Clin Cancer Res 13: 1783–1788.
Becker B, Mullhof G, Farkas B, Wild PJ, Landthaler M, Stoltz W et al. (2004). Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp Dermatol 13: 27–32.
Bijlmakers MJ, Marsh M . (2000). Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance of the Src-kinase p56 (lck). Mol Biol Cell 11: 1585–1595.
Citri A, Harari D, Shohat G, Ramakrishnan P, Gan J, Lavi S et al. (2006). Hsp90 recognises a common surface on client kinases. J Biol Chem 281: 14361–14369.
Connell P, Ballinger CA, Jiang J, Wu Y, Thompson JL, Hohfeld J et al. (2001). The co-chaperone CHIP regulates protein triage decisions mediated by heat shock proteins. Nat Cell Biol 3: 93–96.
Crevecoeur J, Merville MP, Piette J, Gloire G . (2008). Geldanamycin inhibits tyrosine phosphorylation-dependent NF-kB activation. Biochem Pharmacol 75: 2183–2191.
Dai C, Whitesell L . (2005). HSP90: a rising star on the horizon of anticancer targets. Future Oncol 1: 529–540.
Eggert A, Grotzer MA, Ikegaki N, Liu XG, Evans AE, Broduer GM . (2002). Expression of the neurotrophin receptor TrkA down-regulates expression and function of angiogenic stimulators in SH-SY5Y neuroblastoma cells. Cancer Res 62: 1802–1808.
Erdmann F, Jarczowski F, Weiwad M, Fischer G, Edlich F . (2007). Hsp90-mediated inhibition of FKBP38 regulates apoptosis in neuroblastoma cells. FEBS Lett 581: 5709–5714.
Escobar MA, Hoelz DJ, Sandoval JA, Hickey RJ, Grosfeld JL, Malkas LH . (2005). Profiling of nuclear extract proteins from human neuroblastoma cell lines: the search for fingerprints. J Pediatr Surg 40: 349–358.
Frey S, Leskovar A, Reinstein J, Buchner J . (2007). The ATPase cycle of the endoplasmic chaperone Grp94. J Biol Chem 282: 35612–35620.
Geetha T, Wooten MW . (2008). TrkA receptor endolysosomal degradation is both ubiquitin and proteosome dependent. Traffic 9: 1146–1156.
Graner MW, Bigner DD . (2005). Chaperone proteins and brain tumors: potential targets and possible therapeutics. Neuro Oncol 7: 260–277.
Grem JL, Morrison G, Guo XD, Agnew E, Takimoto CH, Thomas R et al. (2005). Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol 23: 1885–1893.
Grenert JP, Johnson BD, Toft DO . (1999). The importance of ATP binding and hydrolysis by Hsp90 in formation and function of protein hetero-complexes. J Biol Chem 274: 17525–17533.
Grenert JP, Sullivan WP, Fadden P, Haystead TAJ, Clark J, Mimnaugh E et al. (1997). The amino acid terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem 272: 23843–23850.
Jakob U, Lilie H, Meyer I, Buchner J . (1995). Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. Implications for heat shock in vivo. J Biol Chem 270: 7288–7294.
Jullien J, Giuli V, Reichardt LF, Rudkin BB . (2002). Molecular kinetics of nerve growth factor receptor trafficking and activation. J Biol Chem 277: 38700–38708.
Kim S, Kang J, Hu W, Evers BM, Chung DH . (2003). Geldanamycin decreases Raf I and Akt levels and induces apoptosis in neuroblastomas. Int J Cancer 103: 352–359.
Lavenius E, Gestblom C, Johansson I, Nanberg E, Pahlman S . (1995). Transfection of TrkA into human neuroblastoma cells restores ability to differentiate in response to nerve growth factor. Cell Growth Differ 6: 727–736.
Lavictoire SJ, Parolin DA, Klimowicz AC, Kelly JF, Lorimer IA . (2003). Interaction of Hsp90 with the nascent form of the mutant epidermal growth factor receptor EGFRvIII. J Biol Chem 278: 5292–5299.
Lòpez-Maderuelo MD, Fernandèz-Renart M, Maratilla C, Renart J . (2001). Opposite effects of the Hsp90 inhibitor geldanamycin: induction of apoptosis in PC12, and differentiation in N2A cells. FEBS Lett 490: 23–27.
Lucarelli E, Kaplan D, Thiele CJ . (1997). Activation of trk-A but not trk-B signal transduction inhibits growth of neuroblastoma cells. Eur J Cancer 33: 2068–2070.
Marcu MG, Doyle M, Berlotti A, Ron D, Hendershot L, Neckers L . (2002). Heat shock protein 90 modulates the unfolded protein reponse by stabilising IRE1α. Mol Cell Biol 22: 8506–8513.
Marczin N, Papapetropuolus A, Catrovas JD . (1993). Tyrosine kinase inhibitors suppress endotoxin- and IL-1β-induced NO synthesis in aortic smooth muscle cells. Am J Physiol 265: 34–43.
Marques C, Pereira P, Taylor F, Liang JN, Reddy VN, Szweda LI et al. (2004). Ubiquitin-dependent lysosomal degradation of the HNE-modified proteins in lens epithelial cells. FASEB J 18: 1424–1426.
Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M . (1989). Molecular and biochemical characterisation of the human trk proto-oncogene. Mol Cell Biol 9: 24–33.
Matei D, Satpathy M, Cao L, Lai YC, Nakashatri H, Donner DB . (2007). The platelet derived growth factor receptor is destabilised by geldanamycins in cancer cells. J Biol Chem 282: 445–453.
Matsushima H, Bogenmann E . (1990). Nerve growth factor (NGF) induces neuronal differentiation in neuroblastoma cells transfected with the NGF receptor cDNA. Mol Cell Biol 10: 5015–5020.
Miyata Y . (2005). The Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents. Curr Pharm Des 11: 1131–1138.
Nakagawara A . (2001). Trk receptor tyrosine kinase: a bridge between cancer and neural development. Cancer Lett 169: 107–114.
Nakagawara A, Arima M, Azar CG, Scavarda NJ, Brodeur GM . (1992). Inverse relationship between trk expression and N-myc amplification in human neuroblastoma. Cancer Res 52: 1364–1368.
Nimmanapalli R, O'Bryan E, Kuhn D, Yamaguchi H, Wang HC, Bhalla KN . (2003). Regulation of 17-AAG-induced apoptosis: role of Bcl-2, BclXl, and Bax downstream of 17-AAG-mediated down-regulation of Akt, Raf-1, and Src kinases. Blood 102: 269–275.
Neckers L . (2002). Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med 8: S55–S61.
Onuoha SC, Mukund SR, Coulstock ET, Sengerova B, Shaw J, Mclaughlin SH et al. (2007). Mechanistic studies on Hsp90 inhibition by ansamycin derivatives. J Mol Biol 372: 287–297.
Orike N, Middleton G, Borthwick E, Buchman V, Cowen T, Davies AM . (2001). Role of PI 3-kinase, Akt and Bcl-2-related proteins in sustaining the survival of neurotrophic factor-independent adult sympathetic neurons. J Cell Biol 154: 995–1005.
Pahlman S, Hedborg F . (2000). Development of the neural crest and sympathetic nervous system. In: Brodeur GM, Sawada T, Tsuchida S, Voute PA (eds). Neuroblastoma. Elsavier Press: Amestadam. pp 9–19.
Peng X, Guo X, Borkan SC, Bharti A, Kuramochi Y, Calderwood S et al. (2005). Heat shock protein 90 stabilisation of ErbB2 expression is disrupted by ATP depletion in myocytes. J Biol Chem 280: 13148–13152.
Puyo S, La Morvan V, Robert J . (2008). Impact of EGFR gene polymorphisms on anticancer drug cytotoxicity in vitro. Mol Diagn Ther 12: 225–234.
Ramanathan RK, Egorin MJ, Eisenman JL, Ramalingham S, Friedland D, Agarwala SS et al. (2007). Phase I and pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with refractory advanced cancers. Clin Cancer Res 13: 1769–1774.
Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokata T et al. (2002). Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett 514: 122–128.
Richter K, Muschler P, Hainzl O, Buchner J . (2001). Coordinated ATP hydrolysis by the Hsp90 dimer. J Biol Chem 276: 33689–33696.
Rosser MF, Trotta BM, Marshall MR, Berwin B, Nicchitta CV . (2004). Adenosine nucleotides and the regulation of GRP94-client protein interaction. Biochemistry 43: 8835–8845.
Scheibel T, Weikl T, Buchner J . (1998). Two chaperone sites in Hsp90 differing in substrate specificity and ATP dependence. Proc Natl Acad Sci USA 95: 1495–1499.
Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N et al. (1996). Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci USA 93: 14536–14541.
Schroder M . (2008). Endoplasmic reticulum stress response. Cell Mol Life Sci 65: 862–894.
Shankovich R, Shue G, Kohtz SD . (1992). Conformational activation of a basic-helix-loop-helix protein (MyoD1) by the c-terminal region of murine Hsp90 (Hsp84). Mol Cell Biol 12: 5059–5068.
Shen JH, Zhang Y, Wu NH, Shen YF . (2007). Resistance to geldanamycin-induced apoptosis in differentiated neuroblastoma SH-SY5Y cells. Neurosci Lett 414: 110–114.
Sidera K, Gaitanou M, Stellas D, Matsas R, Patsavoudi E . (2008). A critical role for Hsp90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J Biol Chem 283: 2031–2041.
Sidera K, Samiotaki M, Yfanti E, Panayotou G, Patsavoudi E . (2004). Involvement of cell surface Hsp90 in cell migration reveals a role in the developing nervous system. J Biol Chem 279: 45379–45388.
Solit DB, Ivy SP, Kopil C, Sikorski R, Morris MJ, Slovin SF et al. (2007). Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res 13: 1775–1782.
Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP . (1997). Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agents. Cell 89: 239–250.
Tacconelli A, Farina AR, Cappabianca L, Cea G, Chioda A, Panella S et al. (2006). Alternative TrkA splicing and cancer. In: Venables JP (ed). Alternative Splicing in Cancer. Transworld Research Network Press: Kerala, India. pp 67–87.
Tacconelli A, Farina AR, Cappabianca L, DeSantis G, Tessitore A, Vetuschi A et al. (2004). TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell 6: 347–360.
Tacconelli A, Farina AR, Cappabianca L, Gulino A, Mackay AR . (2005). Alternative TrkAIII splicing: a potential regulated tumor-promoting switch and therapeutic target in neuroblastoma. Future Med 1: 689–698.
Theodoraki MA, Kunjappu M, Sternberg DW, Caplan AJ . (2007). Akt shows variable sensitivity to an Hsp90 inhibitor depending on cell context. Exp Cell Res 313: 3851–3858.
Vega VL, De Maio A . (2003). Geldanamycin treatment ameliorates the response to LPS in murine macrophages by decreasing CD14 surface expression. Mol Biol Cell 14: 764–773.
Wang Y, Shen J, Arenzana N, Tirasophan W, Kaufman RJ, Pyrwes R . (2000). Activation of ATF6 and an ATF6 binding site by the endoplasmic reticulum stress response. J Biol Chem 275: 27013–27020.
Watson FL, Porcionatto MA, Bhattacharryya A, Stiles CD, Segal RA . (1999). TrkA glycosylation regulates receptor localisation and activity. J Neurobiol 39: 323–326.
Weigel BJ, Blaney SM, Reid JM, Safgren SL, Bagatell R, Kersey J et al. (2007). A phase I study of 17-allyaminogeldanamycin in relapsed/refractory pediatric patients with solid tumors: A Childrens' Oncology Group study. Clin Cancer Res 13: 1789–1793.
Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L . (2002). Chaperon-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci USA 99: 12847–12852.
Xu W, Mimnaugh E, Rosser MFN, Nicchitta C, Marcu M, Yarden Y et al. (2001). Sensitivity of mature ErbB2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem 276: 3702–3708.
Xu W, Neckers L . (2007). Targeting the molecular chaperone heat shock protein 90 provides a multifaceted effect on diverse cell signalling pathways of cancer cells. Clin Cancer Res 13: 1625–1629.
Yoshimura S, Nakamura N, Barr F, Misuri Y, Ikehara Y, Ohno H et al. (2001). Direct targeting of cis-Golgi matrix proteins to the Golgi apparatus. J Cell Sci 114: 4105–4115.
Zhou P, Fernandes N, Dodge IL, Reddi AL, Rao N, Safran H et al. (2003). ErbB2 degradation mediated by the co-chaperone protein CHIP. J Biol Chem 278: 13829–13837.
Acknowledgements
We thank Dr Pyrwes (University of Michigan) for providing the 5xATF6GL3 reporter gene construct, Drs B Cinque, N Di Ianni and M Zani and post-graduate students Paolo Ciufici, Stefania Merolle and Marzia Ragone for technical assistance. This work was supported by AIRC, Progetto Speciale Ministero Della Sanità, MURST-Cofin, MIUR-CNR-Oncology project and Center of Excellence BEMM. We dedicate this work to all the students of L'Aquila University who lost their lives in the earthquake of L'Aquila, 6 April 2009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Farina, A., Tacconelli, A., Cappabianca, L. et al. The neuroblastoma tumour-suppressor TrkAI and its oncogenic alternative TrkAIII splice variant exhibit geldanamycin-sensitive interactions with Hsp90 in human neuroblastoma cells. Oncogene 28, 4075–4094 (2009). https://doi.org/10.1038/onc.2009.256
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.256