Abstract
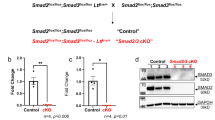
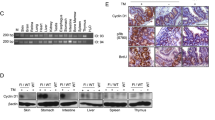
Endometrioid adenocarcinoma is the most frequent form of endometrial cancer, usually developing in pre- and peri-menopausal women. β-catenin abnormalities are common in endometrioid type endometrial carcinomas with squamous differentiation. To investigate the role of β-catenin (Ctnnb1) in uterine development and tumorigenesis, mice were generated which expressed a dominant stabilized β-catenin or had β-catenin conditionally ablated in the uterus by crossing the PRCre mouse with the Ctnnb1f(ex3)/+ mouse or Ctnnb1f/f mouse, respectively. Both of the β-catenin mutant mice have fertility defects and the ability of the uterus to undergo a hormonally induced decidual reaction was lost. Expression of the dominant stabilized β-catenin, PRcre/+Ctnnb1f(ex3)/+, resulted in endometrial glandular hyperplasia, whereas ablation of β-catenin, PRcre/+Ctnnb1f/f, induced squamous cell metaplasia in the murine uterus. Therefore, we have demonstrated that correct regulation of β-catenin is important for uterine function as well as in the regulation of endometrial epithelial differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R . (1997). Beta-catenin is a target for the ubiquitin-proteasome pathway. Embo J 16: 3797–3804.
Anderson MC, Robby SJ, Russell P . (2002). Endometritis, metaplasia, polyp and miscellaneous changes. Churchill Livingstone: London, 285–303 pp.
Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J . (2005). Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 288: 276–283.
Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP et al. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128: 1253–1264.
Buchanan DL, Setiawan T, Lubahn DB, Taylor JA, Kurita T, Cunha GR et al. (1999). Tissue compartment-specific estrogen receptor-alpha participation in the mouse uterine epithelial secretory response. Endocrinology 140: 484–491.
Carta L, Sassoon D . (2004). Wnt7a is a suppressor of cell death in the female reproductive tract and is required for postnatal and estrogen-mediated growth. Biol Reprod 71: 444–454.
Catasus L, Bussaglia E, Rodrguez I, Gallardo A, Pons C, Irving JA et al. (2004). Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol 35: 1360–1368.
Cunha GR, Cooke PS, Kurita T . (2004). Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol 67: 417–434.
Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK et al. (2004). Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol 18: 1238–1250.
Deligdisch L, Holinka CF . (1987). Endometrial carcinoma: two diseases? Cancer Detect Prev 10: 237–246.
Deutscher E, Hung-Chang Yao H . (2007). Essential roles of mesenchyme-derived beta-catenin in mouse Mullerian duct morphogenesis. Dev Biol 307: 227–236.
Di Cristofano A, Ellenson LH . (2007). Endometrial Carcinoma. Annu Rev Pathol 2: 57–85.
Finn CA, Hinchliffe JR . (1964). Reaction Of The Mouse Uterus During Implantation And Deciduoma Formation As Demonstrated By Changes In The Distribution Of Alkaline Phosphatase. J Reprod Fertil 8: 331–338.
Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S . (1998). Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res 58: 3526–3528.
Garcia-Rostan G, Tallini G, Herrero A, D'Aquila TG, Carcangiu ML, Rimm DL . (1999). Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res 59: 1811–1815.
Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R . (1995). Lack of beta-catenin affects mouse development at gastrulation. Development 121: 3529–3537.
Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M et al. (1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J 18: 5931–5942.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT et al. (1998). Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512.
Herington JL, Bi J, Martin JD, Bany BM . (2007). Beta-catenin (CTNNB1) in the mouse uterus during decidualization and the potential role of two pathways in regulating its degradation. J Histochem Cytochem 55: 963–974.
Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR . (2004). Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 164: 577–588.
Hou X, Tan Y, Li M, Dey SK, Das SK . (2004). Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 18: 3035–3049.
Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W . (2000). Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol 148: 567–578.
Huet-Hudson YM, Andrews GK, Dey SK . (1989). Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology 125: 1683–1690.
Ikeda T, Yoshinaga K, Semba S, Kondo E, Ohmori H, Horii A . (2000). Mutational analysis of the CTNNB1 (beta-catenin) gene in human endometrial cancer: frequent mutations at codon 34 that cause nuclear accumulation. Oncol Rep 7: 323–326.
Irving JA, Catasus L, Gallardo A, Bussaglia E, Romero M, Matias-Guiu X et al. (2005). Synchronous endometrioid carcinomas of the uterine corpus and ovary: alterations in the beta-catenin (CTNNB1) pathway are associated with independent primary tumors and favorable prognosis. Hum Pathol 36: 605–619.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ . (2007). Cancer statistics, 2007. CA Cancer J Clin 57: 43–66.
Jha RK, Titus S, Saxena D, Kumar PG, Laloraya M . (2006). Profiling of E-cadherin, beta-catenin and Ca(2+) in embryo-uterine interactions at implantation. FEBS Lett 580: 5653–5660.
Kim YT, Choi EK, Kim JW, Kim DK, Kim SH, Yang WI . (2002). Expression of E-cadherin and alpha-, beta-, gamma-catenin proteins in endometrial carcinoma. Yonsei Med J 43: 701–711.
Kobayashi K, Sagae S, Nishioka Y, Tokino T, Kudo R . (1999). Mutations of the beta-catenin gene in endometrial carcinomas. Jpn J Cancer Res 90: 55–59.
Kong D, Suzuki A, Zou TT, Sakurada A, Kemp LW, Wakatsuki S et al. (1997). PTEN1 is frequently mutated in primary endometrial carcinomas. Nat Genet 17: 143–144.
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW et al. (1997). Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275: 1784–1787.
Koster MI, Dai D, Roop DR . (2007). Conflicting roles for p63 in skin development and carcinogenesis. Cell Cycle 6: 269–273.
Lee KY, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ . (2007a). Mouse models of implantation. Trends Endocrinol Metab 18: 234–239.
Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY et al. (2007b). Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27: 5468–5478.
Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK et al. (2007). Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282: 31725–31732.
Lim H, Ma L, Ma WG, Maas RL, Dey SK . (1999). Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol 13: 1005–1017.
Lin Z, Liu M, Li Z, Kim C, Lee E, Kim I . (2006). DeltaNp63 protein expression in uterine cervical and endometrial cancers. J Cancer Res Clin Oncol 132: 811–816.
Mao TL, Chu JS, Jeng YM, Lai PL, Hsu HC . (2001). Expression of mutant nuclear beta-catenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J Pathol 193: 95–101.
Martin L, Finn CA, Trinder G . (1973). Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol 56: 133–144.
Mericskay M, Kitajewski J, Sassoon D . (2004). Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 131: 2061–2072.
Miller C, Sassoon DA . (1998). Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 125: 3201–3211.
Mirabelli-Primdahl L, Gryfe R, Kim H, Millar A, Luceri C, Dale D et al. (1999). Beta-catenin mutations are specific for colorectal carcinomas with microsatellite instability but occur in endometrial carcinomas irrespective of mutator pathway. Cancer Res 59: 3346–3351.
Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D . (2005). Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci USA 102: 8579–8584.
Moreno-Bueno G, Hardisson D, Sanchez C, Sarrio D, Cassia R, Garcia-Rostan G et al. (2002). Abnormalities of the APC/beta-catenin pathway in endometrial cancer. Oncogene 21: 7981–7990.
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B et al. (1997). Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275: 1787–1790.
Nei H, Saito T, Yamasaki H, Mizumoto H, Ito E, Kudo R . (1999). Nuclear localization of beta-catenin in normal and carcinogenic endometrium. Mol Carcinog 25: 207–218.
Parr BA, McMahon AP . (1998). Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395: 707–710.
Pentecost BT, Newbold RR, Teng CT, McLachlan JA . (1988). Prenatal exposure of male mice to diethylstilbestrol alter the expression of the lactotransferrin gene in seminal vesicles. Mol Endocrinol 2: 1243–1248.
Risinger JI, Hayes AK, Berchuck A, Barrett JC . (1997). PTEN/MMAC1 mutations in endometrial cancers. Cancer Res 57: 4736–4738.
Risinger JI, Hayes K, Maxwell GL, Carney ME, Dodge RK, Barrett JC et al. (1998). PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin Cancer Res 4: 3005–3010.
Romano RA, Birkaya B, Sinha S . (2007). A functional enhancer of keratin14 is a direct transcriptional target of deltaNp63. J Invest Dermatol 127: 1175–1186.
Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I . (2005). Upregulation of TCF4 expression as a transcriptional target of beta-catenin/p300 complexes during trans-differentiation of endometrial carcinoma cells. Lab Invest 85: 768–779.
Saegusa M, Hashimura M, Yoshida T, Okayasu I . (2001). beta-Catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer 84: 209–217.
Saegusa M, Okayasu I . (2001). Frequent nuclear beta-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol 194: 59–67.
Samowitz WS, Powers MD, Spirio LN, Nollet F, van Roy F, Slattery ML . (1999). Beta-catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res 59: 1442–1444.
Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R et al. (1999). The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 96: 5522–5527.
Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ et al. (2005). Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41: 58–66.
Staal FJ, Burgering BM, van de Wetering M, Clevers HC . (1999). Tcf-1-mediated transcription in T lymphocytes: differential role for glycogen synthase kinase-3 in fibroblasts and T cells. Int Immunol 11: 317–323.
Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F et al. (1992). Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359: 76–79.
Sundstrom SA, Komm BS, Ponce-de-Leon H, Yi Z, Teuscher C, Lyttle CR . (1989). Estrogen regulation of tissue-specific expression of complement C3. J Biol Chem 264: 16941–16947.
Tetsu O, McCormick F . (1999). Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426.
Trink B, Osada M, Ratovitski E, Sidransky D . (2007). p63 transcriptional regulation of epithelial integrity and cancer. Cell Cycle 6: 240–245.
Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP . (1999). Female development in mammals is regulated by Wnt-4 signalling. Nature 397: 405–409.
Wappenschmidt B, Wardelmann E, Gehrig A, Schondorf T, Maass N, Bonatz G et al. (2004). PTEN mutations do not cause nuclear beta-catenin accumulation in endometrial carcinomas. Hum Pathol 35: 1260–1265.
Acknowledgements
We thank Jinghua Li and Bryan Ngo for technical assistance; Janet DeMayo, MS for manuscript preparation. This study was supported by the NICHD and, the NIH R01HD042311 and NIH U54HD0077495 (to FJD), NIH R01-CA77530 and the Susan G Komen Award BCTR0503763 (to JPL), NIH 1P50CA098258-01 (to RRB), NIH R01HD057873 and pilot grant from NIH 1P50CA098258-01 (to JWJ), and the NICHD U54HD28934 (to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, JW., Lee, H., Franco, H. et al. β-catenin mediates glandular formation and dysregulation of β-catenin induces hyperplasia formation in the murine uterus. Oncogene 28, 31–40 (2009). https://doi.org/10.1038/onc.2008.363
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.363
Keywords
This article is cited by
-
Risk-stratification machine learning model using demographic factors, gynaecological symptoms and β-catenin for endometrial hyperplasia and carcinoma: a cross-sectional study
BMC Women's Health (2023)
-
The immunolocalization of cadherins and beta-catenin in the cervix and vagina of cycling cows
Veterinary Research Communications (2023)
-
Wnt4, Wnt6 and β-catenin expression in human placental tissue – is there a link with first trimester miscarriage? Results from a pilot study
Reproductive Biology and Endocrinology (2022)
-
Structural and functional changes in rat uterus induced by neonatal androgenization
Journal of Molecular Histology (2022)
-
Molecular characterization of Wdr13 knockout female mice uteri: a model for human endometrial hyperplasia
Scientific Reports (2020)