Abstract
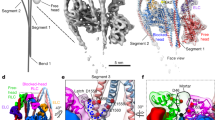
Class I myosins can sense cellular mechanical forces and function as tension-sensitive anchors or transporters. How mechanical load is transduced from the membrane-binding tail to the force-generating head in myosin-1 is unknown. Here we determined the crystal structure of the entire tail of mouse myosin-1c in complex with apocalmodulin, showing that myosin-1c adopts a stable monomer conformation suited for force transduction. The lever-arm helix and the C-terminal extended PH domain of the motor are coupled by a stable post-IQ domain bound to calmodulin in a highly unusual mode. Ca2+ binding to calmodulin induces major conformational changes in both IQ motifs and the post-IQ domain and increases flexibility of the myosin-1c tail. Our study provides a structural blueprint for the neck and tail domains of myosin-1 and expands the target binding modes of the master Ca2+-signal regulator calmodulin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pollard, T.D. & Korn, E.D. Acanthamoeba myosin: I. Isolation from Acanthamoeba castellanii of an enzyme similar to muscle myosin. J. Biol. Chem. 248, 4682–4690 (1973).
McConnell, R.E. & Tyska, M.J. Leveraging the membrane: cytoskeleton interface with myosin-1. Trends Cell Biol. 20, 418–426 (2010).
Hartman, M.A., Finan, D., Sivaramakrishnan, S. & Spudich, J.A. Principles of unconventional myosin function and targeting. Annu. Rev. Cell Dev. Biol. 27, 133–155 (2011).
Hammer, J.A. III & Sellers, J.R. Walking to work: roles for class V myosins as cargo transporters. Nat. Rev. Mol. Cell Biol. 13, 13–26 (2012).
Batters, C. et al. Myo1c is designed for the adaptation response in the inner ear. EMBO J. 23, 1433–1440 (2004).
Stafford, W.F., Walker, M.L., Trinick, J.A. & Coluccio, L.M. Mammalian class I myosin, Myo1b, is monomeric and cross-links actin filaments as determined by hydrodynamic studies and electron microscopy. Biophys. J. 88, 384–391 (2005).
Greenberg, M.J. & Ostap, E.M. Regulation and control of myosin-I by the motor and light chain-binding domains. Trends Cell Biol. 23, 81–89 (2013).
Adams, R.J. & Pollard, T.D. Binding of myosin-I to membrane lipids. Nature 340, 565–568 (1989).
Miyata, H., Bowers, B. & Korn, E.D. Plasma-membrane-association of Acanthamoeba myosin-I. J. Cell Biol. 109, 1519–1528 (1989).
Hayden, S.M., Wolenski, J.S. & Mooseker, M.S. Binding of brush border myosin I to phospholipid vesicles. J. Cell Biol. 111, 443–451 (1990).
Hokanson, D.E., Laakso, J.M., Lin, T., Sept, D. & Ostap, E.M. Myo1c binds phosphoinositides through a putative pleckstrin homology domain. Mol. Biol. Cell 17, 4856–4865 (2006).
Komaba, S. & Coluccio, L.M. Localization of myosin 1b to actin protrusions requires phosphoinositide binding. J. Biol. Chem. 285, 27686–27693 (2010).
Patino-Lopez, G. et al. Myosin 1G is an abundant class I myosin in lymphocytes whose localization at the plasma membrane depends on its ancient divergent pleckstrin homology (PH) domain (Myo1PH). J. Biol. Chem. 285, 8675–8686 (2010).
Krendel, M., Osterweil, E.K. & Mooseker, M.S. Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis. FEBS Lett. 581, 644–650 (2007).
Holt, J.R. et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108, 371–381 (2002).
Bose, A. et al. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420, 821–824 (2002).
Tiwari, A., Jung, J.J., Inamdar, S.M., Nihalani, D. & Choudhury, A. The myosin motor Myo1c is required for VEGFR2 delivery to the cell surface and for angiogenic signaling. Am. J. Physiol. Heart Circ. Physiol. 304, H687–H696 (2013).
Arif, E. et al. Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol. Cell. Biol. 31, 2134–2150 (2011).
Fan, Y., Eswarappa, S.M., Hitomi, M. & Fox, P.L. Myo1c facilitates G-actin transport to the leading edge of migrating endothelial cells. J. Cell Biol. 198, 47–55 (2012).
Boguslavsky, S. et al. Myo1c binding to submembrane actin mediates insulin-induced tethering of GLUT4 vesicles. Mol. Biol. Cell 23, 4065–4078 (2012).
Jontes, J.D., Wilson-Kubalek, E.M. & Milligan, R.A. A 32° tail swing in brush border myosin I on ADP release. Nature 378, 751–753 (1995).
Jontes, J.D. & Milligan, R.A. Brush border myosin-I structure and ADP-dependent conformational changes revealed by cryoelectron microscopy and image analysis. J. Cell Biol. 139, 683–693 (1997).
Kollmar, M., Durrwang, U., Kliche, W., Manstein, D.J. & Kull, F.J. Crystal structure of the motor domain of a class-I myosin. EMBO J. 21, 2517–2525 (2002).
Münnich, S., Taft, M.H. & Manstein, D.J. Crystal structure of human myosin 1c—the motor in GLUT4 exocytosis: implications for Ca2+ regulation and 14-3-3 binding. J. Mol. Biol. 426, 2070–2081 (2014).
Shuman, H. et al. A vertebrate myosin-I structure reveals unique insights into myosin mechanochemical tuning. Proc. Natl. Acad. Sci. USA 111, 2116–2121 (2014).
Kretsinger, R.H. & Nockolds, C.E. Carp muscle calcium-binding protein: II. Structure determination and general description. J. Biol. Chem. 248, 3313–3326 (1973).
Zhang, M., Tanaka, T. & Ikura, M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 2, 758–767 (1995).
Kuboniwa, H. et al. Solution structure of calcium-free calmodulin. Nat. Struct. Biol. 2, 768–776 (1995).
Finn, B.E. et al. Calcium-induced structural-changes and domain autonomy in calmodulin. Nat. Struct. Biol. 2, 777–783 (1995).
Babu, Y.S. et al. Three-dimensional structure of calmodulin. Nature 315, 37–40 (1985).
Ikura, M. et al. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science 256, 632–638 (1992).
Hoeflich, K.P. & Ikura, M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell 108, 739–742 (2002).
Terrak, M., Wu, G.M., Stafford, W.F., Lu, R.C. & Dominguez, R. Two distinct myosin light chain structures are induced by specific variations within the bound IQ motifs: functional implications. EMBO J. 22, 362–371 (2003).
Houdusse, A. et al. Crystal structure of apo-calmodulin bound to the first two IQ motifs of myosin V reveals essential recognition features. Proc. Natl. Acad. Sci. USA 103, 19326–19331 (2006).
Terrak, M., Rebowski, G., Lu, R.C., Grabarek, Z. & Dominguez, R. Structure of the light chain-binding domain of myosin V. Proc. Natl. Acad. Sci. USA 102, 12718–12723 (2005).
Adamek, N., Coluccio, L.M. & Geeves, M.A. Calcium sensitivity of the cross-bridge cycle of Myo1c, the adaptation motor in the inner ear. Proc. Natl. Acad. Sci. USA 105, 5710–5715 (2008).
Peng, A.W., Effertz, T. & Ricci, A.J. Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron 80, 960–972 (2013).
Chen, X.W., Leto, D., Chiang, S.H., Wang, Q. & Saltiel, A.R. Activation of RaIA is required for insulin-stimulated glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev. Cell 13, 391–404 (2007).
Lewis, J.H., Greenberg, M.J., Laakso, J.M., Shuman, H. & Ostap, E.M. Calcium regulation of myosin-I tension sensing. Biophys. J. 102, 2799–2807 (2012).
Zhu, T., Sata, M. & Ikebe, M. Functional expression of mammalian myosin Iβ: analysis of its motor activity. Biochemistry 35, 513–522 (1996).
Manceva, S. et al. Calcium regulation of calmodulin binding to and dissociation from the myo1c regulatory domain. Biochemistry 46, 11718–11726 (2007).
Lieto-Trivedi, A. & Coluccio, L.M. Calcium, nucleotide, and actin affect the interaction of mammalian Myo1c with its light chain calmodulin. Biochemistry 47, 10218–10226 (2008).
Mazzolini, R. et al. Brush border Myosin Ia has tumor suppressor activity in the intestine. Proc. Natl. Acad. Sci. USA 109, 1530–1535 (2012).
Tokuo, H. & Coluccio, L.M. Myosin-1c regulates the dynamic stability of E-cadherin-based cell-cell contacts in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 24, 2820–2833 (2013).
Gillespie, P.G. & Cyr, J.L. Calmodulin binding to recombinant myosin-1c and myosin-1c IQ peptides. BMC Biochem. 3, 31 (2002).
Whittaker, M. & Milligan, R.A. Conformational changes due to calcium-induced calmodulin dissociation in brush border myosin I-decorated F-actin revealed by cryoelectron microscopy and image analysis. J. Mol. Biol. 269, 548–557 (1997).
Greenberg, M.J., Lin, T.M., Goldman, Y.E., Shuman, H. & Ostap, M. Myosin IC generates power over a range of loads via a new tension-sensing mechanism. Proc. Natl. Acad. Sci. USA 109, E2433–E2440 (2012).
Laakso, J.M., Lewis, J.H., Shuman, H. & Ostap, E.M. Myosin I can act as a molecular force sensor. Science 321, 133–136 (2008).
DeMaria, C.D., Soong, T.W., Alseikhan, B.A., Alvania, R.S. & Yue, D.T. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature 411, 484–489 (2001).
Grabarek, Z. Structural basis for diversity of the EF-hand calcium-binding proteins. J. Mol. Biol. 359, 509–525 (2006).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Vonrhein, C., Blanc, E., Roversi, P. & Bricogne, G. Automated structure solution with autoSHARP. Methods Mol. Biol. 364, 215–230 (2007).
Komander, D. et al. Structural insights into the regulation of PDK1 by phosphoinositides and inositol phosphates. EMBO J. 23, 3918–3928 (2004).
Vagin, A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 (1997).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Mccoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Acknowledgements
We thank the BL17U station at the Shanghai Synchrotron Radiation Facility for X-ray beam time; Y. Zhang for technical help; and members of the Zhang laboratory for comments on the manuscript. This work was supported by grants from the Research Grant Council of Hong Kong (663811, 663812, 664113, HKUST6/CRF/10, SEG_HKUST06, T13-607/12R and AoE/M09/12 to M.Z.) and the National Key Basic Research Program of China (2014CB910204 to M.Z.). We thank C. Petit (Institut Pasteur) for the full-length Myo1c.
Author information
Authors and Affiliations
Contributions
Q.L., J.L. and M.Z. designed experiments and analyzed data. Q.L., J.L. and F.Y. performed experiments. Q.L., J.L. and M.Z. wrote the manuscript. M.Z. coordinated the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Myo1c tail binds to three apo-CaM.
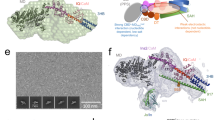
(a) Sedimentation velocity curves fitting results of myo1c fragments with saturated amount of apo-CaM. IQ1-end and IQ2-end do not contain any fusion tags. CaM in complex with IQ3-end contained a Trx fusion tag, which was used to facilitate the purification of the complex. (b) The molecular masses of various Myo1c fragments with saturated amount of apo-CaM derived from sedimentation velocity analysis (the second column). Black star: the “IQ3-end” protein was purified in the Trx-fused form, and the mass of fusion tag was subtracted from “IQ3-end” for easy comparison. The measured molecular mass of “IQ1-end” is ~10 kD larger than the theoretical value, this discrepancy is likely due to the fitting error caused by friction ratio increase for the highly elongated shape of the complex. (c) The molecular masses of three Myo1c fragments, each saturated with apo-CaM, derived from FPLC coupled with static light scattering experiments (indicated on the top of each peak). The theoretical masses for the IQ1-852–(CaM)3, IQ2-852–(CaM)2 and IQ3-852–CaM are 68.6 kD, 49.1 kD and 29.8 kD, respectively.
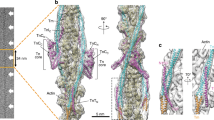
Supplementary Figure 2 Electron density maps of the myo1c tail and detailed interactions of the myo1c post-IQ region with the N-terminal IQ3 motif and the C-terminal extended PH domain.
(a) Overview of the experimental determined Au positions (magenta, σ=4) and the phased electron density (blue, σ=1). (b) 2Fo-Fc electron density map (σ=1) of the whole tail. (c) Stereo views of 2Fo-Fc electron density map (Left, σ=1) and ribbon diagram (Right) of the post-IQ region in complex with N-lobe of CaM3. (d) 2Fo-Fc electron density map (Left, σ=1) and ribbon diagram (Right) of the extended PH domain. (e) The interface between the lever arm and post-IQ is mainly composed of hydrophobic interactions between IQ3 and α2. For clarity, CaM is not shown. (f) The interaction between post-IQ and the extended PH domain is mediated by hydrogen bonds and hydrophobic interactions between α4 of post-IQ and α5/α7 of the extended PH domain.
Supplementary Figure 3 Sequence alignment of the neck-tail domain of myo1c from vertebrates and different members of the class I human myosins.
Identical and highly similar residues are colored. The secondary structure elements are labeled using the same color coding scheme shown in Fig. 1b.
Supplementary Figure 4 NMR-based validation of the post-IQ–CaM interaction.
(a) 15N-HSQC spectra of 15N-labeled IQ3-852 in complex with 15N-labeled WT-CaM or with 15N-labeled F16C-CaM. The overlap of most of the peaks of the two spectra indicates that the F16C mutation of CaM does not alter the overall structure of the complex between CaM and IQ3-852. (b) 15N-HSQC spectra of 15N-labeled apo-CaM titrated with the IQ3 peptide. Color coding of the peaks corresponds to different apo-CaM/IQ3 ratios indicated in the figure. For clarity, only a few representative peaks are labeled. Peaks from C lobe that change are highlighted with black boxes.
Supplementary Figure 5 Flexibility change of Myo1c–CaM complex upon addition of Ca2+
(a&b) Time-dependent, partial trypsin digestions of Myo1c IQ1-end (a) and IQ2-end (b) in the absence or presence of Ca2+. Myo1c-CaM complexes were incubated in a digestion buffer with or without calcium (1 mM EDTA/CaCl2, 50 mM Tris PH 7.8, 100 mM NaCl, and 1 mM DTT) containing 1% trypsin (i.e. at a trypsin:Myo1c mass ratio of 1:100). Samples were withdrawn at 0, 1, 5, 15, 30 min after mixing with trypsin for SDS-PAGE analysis using Coomassie Blue staining. (c) Thrombin digestions of Myo1c IQ1-end (left) and IQ2-end (right) in the absence and presence of Ca2+. Myo1c-CaM complexes were cleaved with thrombin at 10 units/mg of complex for 10 h at 37 °C under the same buffer conditions as those in panels a&b. (d) CD spectra of Myo1c IQ2-852 in complex with calmodulin with or without Ca2+. To make sure that the relatively small ellipticity changes are reliable, an aliquot of apo-CaM-bound Myo1c IQ2-852 at a concentration of 4 μM complex (in the buffer of 1 mM EDTA, 50 mM Tris PH 7.8, 100 mM NaCl, and 1 mM DTT) was split into two equal portions. A few μl of concentrated CaCl2 was added to one sample to make the final Ca2+ to 2 mM, and the same volume of H2O was added to the other sample to make sure that the protein concentrations of the two samples for the CD experiments are identical. Upon addition of Ca2+, the ellipticity of IQ2-853 decreases (red curve). It has been well established that binding of Ca2+ to CaM stabilizes helical structures of the protein and thus leads to increase of the ellipticity. The decrease of the helical content of the IQ2-852 and CaM complex upon addition of Ca2+ is most likely resulted from the loss or destabilization of the α-helices from the Myo1c tail.
Supplementary Figure 6 Binding of IQ2-852 and IQ2-829 to Ca2+-CaM.
FPLC coupled with static light scattering analysis of the bindings of IQ2-852 (red line) and IQ2-829 (blue line) to saturated amount of Ca2+-CaM. All samples can be fitted with two CaM molecules bound to the Myo1c fragments, showing that α4 of post-IQ does not involve in binding to Ca2+-CaM.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1371 kb)
Rights and permissions
About this article
Cite this article
Lu, Q., Li, J., Ye, F. et al. Structure of myosin-1c tail bound to calmodulin provides insights into calcium-mediated conformational coupling. Nat Struct Mol Biol 22, 81–88 (2015). https://doi.org/10.1038/nsmb.2923
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2923
This article is cited by
-
MYO1H is a novel candidate gene for autosomal dominant pure hereditary spastic paraplegia
Molecular Genetics and Genomics (2022)
-
SH3BGRL3 binds to myosin 1c in a calcium dependent manner and modulates migration in the MDA-MB-231 cell line
BMC Molecular and Cell Biology (2021)
-
Vertebrate myosin 1d regulates left–right organizer morphogenesis and laterality
Nature Communications (2018)