Abstract
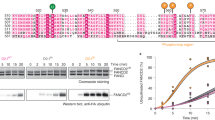
In response to DNA damage or replication fork stress, the Fanconi anemia pathway is activated, leading to monoubiquitination of FANCD2 and FANCI and their colocalization in foci. Here we show that, in the chicken DT40 cell system, multiple alanine-substitution mutations in six conserved and clustered Ser/Thr-Gln motifs of FANCI largely abrogate monoubiquitination and focus formation of both FANCI and FANCD2, resulting in loss of DNA repair function. Conversely, FANCI carrying phosphomimic mutations on the same six residues induces constitutive monoubiquitination and focus formation of FANCI and FANCD2, and protects against cell killing and chromosome breakage by DNA interstrand cross-linking agents. We propose that the multiple phosphorylation of FANCI serves as a molecular switch in activation of the Fanconi anemia pathway. Mutational analysis of putative phosphorylation sites in human FANCI indicates that this switch is evolutionarily conserved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Hoeijmakers, J.H. Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374 (2001).
Nijnik, A. et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature 447, 686–690 (2007).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997–1002 (2004).
Rossi, D.J. et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447, 725–729 (2007).
D'Andrea, A.D. & Grompe, M. The Fanconi anaemia/BRCA pathway. Nat. Rev. Cancer 3, 23–34 (2003).
Wang, W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat. Rev. Genet. 8, 735–748 (2007).
Smogorzewska, A. et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell 129, 289–301 (2007).
Sims, A.E. et al. FANCI is a second monoubiquitinated member of the Fanconi anemia pathway. Nat. Struct. Mol. Biol. 14, 564–567 (2007).
Dorsman, J.C. et al. Identification of the Fanconi anemia complementation group I gene, FANCI. Cell. Oncol. 29, 211–218 (2007).
Andreassen, P.R., D'Andrea, A.D. & Taniguchi, T. ATR couples FANCD2 monoubiquitination to the DNA-damage response. Genes Dev. 18, 1958–1963 (2004).
Machida, Y.J. et al. UBE2T is the E2 in the Fanconi anemia pathway and undergoes negative autoregulation. Mol. Cell 23, 589–596 (2006).
Ciccia, A. et al. Identification of FAAP24, a Fanconi anemia core complex protein that interacts with FANCM. Mol. Cell 25, 331–343 (2007).
Ling, C. et al. FAAP100 is essential for activation of the Fanconi anemia-associated DNA damage response pathway. EMBO J. 26, 2104–2114 (2007).
Taniguchi, T. et al. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood 100, 2414–2420 (2002).
Matsushita, N. et al. A FancD2-monoubiquitin fusion reveals hidden functions of Fanconi anemia core complex in DNA repair. Mol. Cell 19, 841–847 (2005).
Nijman, S.M. et al. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol. Cell 17, 331–339 (2005).
Howlett, N.G. et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297, 606–609 (2002).
Xia, B. et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol. Cell 22, 719–729 (2006).
Cantor, S.B. et al. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell 105, 149–160 (2001).
Levitus, M. et al. Heterogeneity in Fanconi anemia: evidence for two new genetic subtypes. Blood 103, 2498–2503 (2004).
Seki, S. et al. A requirement of FancL and FancD2 monoubiquitination in DNA repair. Genes Cells 12, 299–310 (2007).
Yamamoto, K. et al. Fanconi anemia protein FANCD2 promotes immunoglobulin gene conversion and DNA repair through a mechanism related to homologous recombination. Mol. Cell. Biol. 25, 34–43 (2005).
Hirano, S. et al. Functional relationships of FANCC to homologous recombination, translesion synthesis, and BLM. EMBO J. 24, 418–427 (2005).
Matsuoka, S. et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160–1166 (2007).
Kinoshita, E., Kinoshita-Kikuta, E., Takiyama, K. & Koike, T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics 5, 749–757 (2006).
Zou, L. & Elledge, S.J. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300, 1542–1548 (2003).
Sarkaria, J.N. et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59, 4375–4382 (1999).
Oestergaard, V.H. et al. Deubiquitination of FANCD2 is required for DNA crosslink repair. Mol. Cell 28, 798–809 (2007).
West, S.C. Molecular views of recombination proteins and their control. Nat. Rev. Mol. Cell Biol. 4, 435–445 (2003).
Ho, G.P., Margossian, S., Taniguchi, T. & D'Andrea, A.D. Phosphorylation of FANCD2 on two novel sites is required for mitomycin C resistance. Mol. Cell. Biol. 26, 7005–7015 (2006).
Wang, X. et al. Chk1-mediated phosphorylation of FANCE is required for the Fanconi anemia/BRCA pathway. Mol. Cell. Biol. 27, 3098–3108 (2007).
Meetei, A.R. et al. A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M. Nat. Genet. 37, 958–963 (2005).
Hunter, T. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol. Cell 28, 730–738 (2007).
Harper, J.W. & Elledge, S.J. The DNA damage response: ten years after. Mol. Cell 28, 739–745 (2007).
Yamamoto, K. et al. Fanconi anemia FANCG protein in mitigating radiation- and enzyme-induced DNA double-strand breaks by homologous recombination in vertebrate cells. Mol. Cell. Biol. 23, 5421–5430 (2003).
Ishiai, M. et al. DNA cross-link repair protein SNM1A interacts with PIAS1 in nuclear focus formation. Mol. Cell. Biol. 24, 10733–10741 (2004).
Kitao, H. et al. Functional interplay between BRCA2/FANCD1 and FANCC in DNA repair. J. Biol. Chem. 281, 21312–21320 (2006).
Tomida, J., Kitao, H., Kinoshita, E. & Takata, M. Detection of phosphorylation on large proteins by western blotting using Phos-tag containing gel. Nat. Protocols, 10.1038/nprot.2008.232 (in the press).
Acknowledgements
We would like to thank T. Natsume (National Institute of Advanced Industrial Science and Technology, Japan), K. Komatsu (Kyoto University), H. Kurumizaka (Waseda University), J. Lin and J.W. Harper (Harvard Medical School) for reagents; R. Sasaki (Digital Microsystems) for advice and help in microscopy; K. Namikoshi for expert technical help; and H. Shimamoto and S. Arai for secretarial assistance. This work was supported in part by Grants-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan (M.T.), by grants from Ministry of Health, Labour, and Welfare (M.T.), and by US National Institute of Allergy and Infectious Diseases grant 1U19A1067751 and US National Institutes of Health grants (S.J.E.). A. Smogorzewska is supported by T32CA09216 to the MGH Pathology Department. Financial support was also provided by The Novartis Foundation (Japan) for the Promotion of Science (M.T.) and The Uehara Memorial Foundation (M.T.). S.J.E. is funded by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
M.T., A. Smogorzewska and S.J.E. conceived and designed the experiments; M.I., H.K., A. Saberi and E.U. performed most of the DT40 cell experiments; M.I., A.K. and S.T. performed microscopic analysis; E.K., E.K.-K., T.K. and J.T. designed and performed Phos-tag experiments; A. Smogorzewska performed human cell experiments; M.I. and H.K. analyzed the DT40 cell data; M.T., A. Smogorzewska and S.J.E. wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 11181 kb)
Rights and permissions
About this article
Cite this article
Ishiai, M., Kitao, H., Smogorzewska, A. et al. FANCI phosphorylation functions as a molecular switch to turn on the Fanconi anemia pathway. Nat Struct Mol Biol 15, 1138–1146 (2008). https://doi.org/10.1038/nsmb.1504
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1504
This article is cited by
-
The key to the FANCD2–FANCI lock
Nature Structural & Molecular Biology (2022)
-
Fanconi anemia: current insights regarding epidemiology, cancer, and DNA repair
Human Genetics (2022)
-
A functionally impaired missense variant identified in French Canadian families implicates FANCI as a candidate ovarian cancer-predisposing gene
Genome Medicine (2021)
-
Fanconi anemia pathway as a prospective target for cancer intervention
Cell & Bioscience (2020)
-
FANCD2–FANCI is a clamp stabilized on DNA by monoubiquitination of FANCD2 during DNA repair
Nature Structural & Molecular Biology (2020)