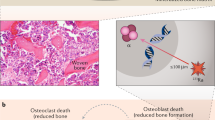
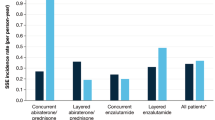
Key Points
-
Bone metastases are common in metastatic castration-resistant prostate cancer (mCRPC) and lead to skeletal-related events (SREs), which are a major cause of patient disability, reduced quality of life, and death
-
Understanding the pathophysiology of bone metastasis resulted in the approval of agents that improve clinical outcomes in patients with mCRPC, and new targeted agents are under development in this setting
-
Managing both the tumour and associated SREs is important to improve survival and quality of life of patients with mCRPC
-
Osteoclast inhibitors are part of the standard treatment of CRPC metastatic to bone; denosumab has been shown to reduce skeletal morbidity more than zoledronic acid
-
New antitumour agents increase survival and some, such as abiraterone and enzalutamide and 223Ra, also decrease skeletal morbidity
-
Ongoing clinical studies are investigating the optimal position and combinations of approved agents in the treatment paradigm to maximize patient benefit in this setting
Abstract
Bone metastases develop in most patients with metastatic castration-resistant prostate cancer (mCRPC). They affect the structural integrity of bone, manifesting as pain and skeletal-related events (SREs), and are the primary cause of patient disability, reduced quality of life (QOL) and death. Understanding the pathophysiology of bone metastases resulted in the development of agents that improve clinical outcome, suggesting that managing both the systemic disease and associated bone events is important. Historically, the treatment of CRPC bone metastases with early radiopharmaceuticals and external beam radiation therapy was largely supportive; however, now, zoledronic acid and denosumab are integral to the therapeutic strategy for mCRPC. These agents substantially reduce skeletal morbidity and improve patient QOL. Radium-223 dichloride is the first bone-targeting agent to show improved survival and reduced pain and symptomatic skeletal events in patients with mCRPC without visceral disease. Five other systemic agents are currently approved for use in mCRPC based on their ability to improve survival. These include the cytotoxic drugs docetaxel and cabazitaxel, the hormone-based therapies, abiraterone and enzalutamide, and the immunotherapeutic vaccine sipuleucel-T. Abiraterone and enzalutamide are able to reduce SREs and improve survival in this setting. Novel agents targeting tumour and bone cells are under clinical development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ferlay, J. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 International Agency for Research on Cancer [online], (2013).
Horwich, A., Parker, C., de Reijke, T. & Kataja, V. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24 (Suppl. 6), 106–114 (2013).
National Comprehensive Cancer Network®. NCCN Guidelines for Patients: Prostate Cancer Version 1.2014 [online], (2015).
Scher, H. I., Buchanan, G., Gerald, W., Butler, L. M. & Tilley, W. D. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr. Relat. Cancer 11, 459–476 (2004).
Bubendorf, L. et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum. Pathol. 31, 578–583 (2000).
Scher, H. I. & Chung, L. W. Bone metastases: improving the therapeutic index. Semin. Oncol. 21, 630–656 (1994).
Keller, E. T. et al. Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev. 20, 333–349 (2001).
Weinfurt, K. P. et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann. Oncol. 16, 579–584 (2005).
Sabbatini, P. et al. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J. Clin. Oncol. 17, 948–957 (1999).
Nørgaard, M. et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J. Urol. 184, 162–167 (2010).
Petrylak, D. P. et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 351, 1513–1520 (2004).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Fizazi, K. et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 13, 983–992 (2012).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Fizazi, K. et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 15, 1147–1156 (2014).
Parker, C. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369, 213–223 (2013).
Saad, F. et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl Cancer Inst. 96, 879–882 (2004).
Saad, F. et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl Cancer Inst. 94, 1458–1468 (2002).
Fizazi, K. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377, 813–822 (2011).
Rubini, G., Nicoletti, A., Rubini, D. & Asabella, A. N. Radiometabolic treatment of bone-metastasizing cancer: from 186rhenium to 223Radium. Cancer Biother. Radiopharm. 29, 1–11 (2014).
Goyal, J. & Antonarakis, E. S. Bone-targeting radiopharmaceuticals for the treatment of prostate cancer with bone metastases. Cancer Lett. 323, 135–146 (2012).
Sartor, O. et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 15, 738–746 (2014).
Nilsson, S. et al. Pain analysis from the phase III randomized ALSYMPCA study with radium-223 dichloride (Ra-223) in patients with castration-resistant prostate cancer (CRPC) with bone metastases [abstract]. J. Clin. Oncol. 31 (Suppl. 6), 19 (2013).
Wang, C. & Shen, Y. Study on the distribution features of bone metastases in prostate cancer. Nucl. Med. Commun. 33, 379–383 (2012).
Wang, C. Y., Wu, G. Y., Shen, M. J., Cui, K. W. & Shen, Y. Comparison of distribution characteristics of metastatic bone lesions between breast and prostate carcinomas. Oncol. Lett. 5, 391–397 (2013).
Conti, G. et al. Prostate cancer metastases to bone: observational study for the evaluation of clinical presentation, course and treatment patterns. Presentation of the METAURO protocol and of patient baseline features. Arch. Ital. Urol. Androl. 80, 59–64 (2008).
Smith, M. R. et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann. Oncol. 26, 368–374 (2015).
von Moos, R., Sternberg, C., Body, J. J. & Bokemeyer, C. Reducing the burden of bone metastases: current concepts and treatment options. Support. Care Cancer 21, 1773–1783 (2013).
Halabi, S. et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J. Clin. Oncol. 26, 2544–2549 (2008).
Oefelein, M. G., Ricchiuti, V., Conrad, W. & Resnick, M. I. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J. Urol. 168, 1005–1007 (2002).
DePuy, V. et al. Effects of skeletal morbidities on longitudinal patient-reported outcomes and survival in patients with metastatic prostate cancer. Support. Care Cancer 15, 869–876 (2007).
Hagiwara, M., Delea, T. E., Saville, M. W. & Chung, K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 16, 23–27 (2013).
Body, J. J., Chevalier, P., Gunther, O., Hechmati, G. & Lamotte, M. The economic burden associated with skeletal-related events in patients with bone metastases secondary to solid tumors in Belgium. J. Med. Econ. 16, 539–546 (2013).
Jayasekera, J. et al. The economic burden of skeletal-related events among elderly men with metastatic prostate cancer. Pharmacoeconomics 32, 173–191 (2014).
Lipton, A. Bisphosphonates and breast carcinoma: present and future. Cancer 88, 3033–3037 (2000).
Ibrahim, T. et al. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer 116, 1406–1418 (2010).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Trouvin, A. P. & Goëb, V. Receptor activator of nuclear factor-κB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin. Interv. Aging 5, 345–354 (2010).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573 (1889).
Casimiro, S., Guise, T. A. & Chirgwin, J. The critical role of the bone microenvironment in cancer metastases. Mol. Cell. Endocrinol. 310, 71–81 (2009).
Wang, J., Loberg, R. & Taichman, R. S. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev. 25, 573–587 (2006).
Taichman, R. S. et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 62, 1832–1837 (2002).
Barthel, S. R. et al. Definition of molecular determinants of prostate cancer cell bone extravasation. Cancer Res. 73, 942–952 (2013).
Jones, D. H. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692–696 (2006).
Chu, G. C. et al. RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocr. Relat. Cancer 21, 311–326 (2014).
Yin, J. J. et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl Acad. Sci. USA 100, 10954–10959 (2003).
Pinzone, J. J. et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 113, 517–525 (2009).
Guise, T. A. et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 12, 6213s–6216s (2006).
Buijs, J. T., Stayrook, K. R. & Guise, T. A. TGF-β in the bone microenvironment: role in breast cancer metastases. Cancer Microenviron. 4, 261–281 (2011).
Maeda, S., Hayashi, M., Komiya, S., Imamura, T. & Miyazono, K. Endogenous TGF-β signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 23, 552–563 (2004).
Clines, G. A. & Guise, T. A. Molecular mechanisms and treatment of bone metastasis. Expert Rev. Mol. Med. 10, e7 (2008).
Stewart, A. F. PTHrP(1–36) as a skeletal anabolic agent for the treatment of osteoporosis. Bone 19, 303–306 (1996).
Morrissey, C. et al. Effects of androgen deprivation therapy and bisphosphonate treatment on bone in patients with metastatic castration-resistant prostate cancer: results from the University of Washington Rapid Autopsy Series. J. Bone Miner. Res. 28, 333–340 (2013).
Coleman, R., Body, J. J., Aapro, M., Hadji, P. & Herrstedt, J. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 25 (Suppl. 3), 124–137 (2014).
Yonou, H. et al. Intraosseous growth of human prostate cancer in implanted adult human bone: relationship of prostate cancer cells to osteoclasts in osteoblastic metastatic lesions. Prostate 58, 406–413 (2004).
Keller, E. T. & Brown, J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J. Cell. Biochem. 91, 718–729 (2004).
Cook, L. M., Shay, G., Araujo, A. & Lynch, C. C. Integrating new discoveries into the “vicious cycle” paradigm of prostate to bone metastases. Cancer Metastasis Rev. 33, 511–525 (2014).
Frieling, J. S., Basanta, D. & Lynch, C. C. Current and emerging therapies for bone metastatic castration-resistant prostate cancer. Cancer Control 22, 109–120 (2015).
Ell, B. et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 24, 542–556 (2013).
Deng, X. et al. Recent advances in bone-targeted therapies of metastatic prostate cancer. Cancer Treat. Rev. 40, 730–738 (2014).
Dayyani, F., Gallick, G. E., Logothetis, C. J. & Corn, P. G. Novel therapies for metastatic castrate-resistant prostate cancer. J. Natl Cancer Inst. 103, 1665–1675 (2011).
Muralidharan, A. & Smith, M. T. Pathobiology and management of prostate cancer-induced bone pain: recent insights and future treatments. Inflammopharmacology 21, 339–363 (2013).
Falk, S. & Dickenson, A. H. Pain and nociception: mechanisms of cancer-induced bone pain. J. Clin. Oncol. 32, 1647–1654 (2014).
Neville-Webbe, H. L. & Coleman, R. E. Bisphosphonates and RANK ligand inhibitors for the treatment and prevention of metastatic bone disease. Eur. J. Cancer 46, 1211–1222 (2010).
Roelofs, A. J., Thompson, K., Gordon, S. & Rogers, M. J. Molecular mechanisms of action of bisphosphonates: current status. Clin. Cancer Res. 12, 6222s–6230s (2006).
Green, J. R., Müller, K. & Jaeggi, K. A. Preclinical pharmacology of CGP 42'446, a new, potent, heterocyclic bisphosphonate compound. J. Bone Miner. Res. 9, 745–751 (1994).
Body, J. J. Clinical research update: zoledronate. Cancer 80 (Suppl. 8), 1699–1701 (1997).
Berenson, J. R. et al. A phase I dose-ranging trial of monthly infusions of zoledronic acid for the treatment of osteolytic bone metastases. Clin. Cancer Res. 7, 478–485 (2001).
Body, J. J., Lortholary, A., Romieu, G., Vigneron, A. M. & Ford, J. A dose-finding study of zoledronate in hypercalcemic cancer patients. J. Bone Miner. Res. 14, 1557–1561 (1999).
Smith, M. R. et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J. Clin. Oncol. 32, 1143–1150 (2014).
Wirth, M. et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur. Urol. 67, 482–491 (2015).
Small, E. J., Smith, M. R., Seaman, J. J., Petrone, S. & Kowalski, M. O. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J. Clin. Oncol. 21, 4277–4284 (2003).
Ernst, D. S. et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J. Clin. Oncol. 21, 3335–3342 (2003).
Kostenuik, P. J. et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J. Bone Miner. Res. 24, 182–195 (2009).
Body, J. J. et al. A study of the biological receptor activator of nuclear factor-κB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin. Cancer Res. 12, 1221–1228 (2006).
Lipton, A. et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J. Clin. Oncol. 25, 4431–4437 (2007).
Fizazi, K. et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. 27, 1564–1571 (2009).
Body, J. J. et al. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J. Bone Miner. Res. 25, 440–446 (2010).
US Food and Drug Administration. Highlights of prescribing information, XGEVA (denosumab) injection, for subcutaneous use [online], (2010).
European Medicines Agency. Xgeva: EPAR - Product Information [online], (2014).
Body, J. J. Dosing regimens and main adverse events of bisphosphonates. Semin. Oncol. 28, 49–53 (2001).
European Medicines Agency. Zometa: EPAR - Product Information [online], (2015).
Drugs.com. Zometa - FDA prescribing information, side effects and uses [online], (2015).
Saylor, P. J., Lee, R. J. & Smith, M. R. Emerging therapies to prevent skeletal morbidity in men with prostate cancer. J. Clin. Oncol. 29, 3705–3714 (2011).
Lipton, A. et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. Cancer 48, 3082–3092 (2012).
Body, J. J. Bisphosphonates for malignancy-related bone disease: current status, future developments. Support. Care Cancer 14, 408–418 (2006).
Saad, F. et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 23, 1341–1347 (2012).
Khan, A. A. et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J. Bone Miner. Res. 30, 3–23 (2015).
Porter, A. T. et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 25, 805–813 (1993).
Bruland, Ø. S., Nilsson, S., Fisher, D. R. & Larsen, R. H. High-linear energy transfer irradiation targeted to skeletal metastases by the α-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin. Cancer Res. 12, 6250s–6257s (2006).
Buchali, K. et al. Results of a double blind study of 89-strontium therapy of skeletal metastases of prostatic carcinoma. Eur. J. Nucl. Med. 14, 349–51 (1988).
Tu, S. M. et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 357, 336–341 (2001).
Lewington, V. J. et al. A prospective, randomised double-blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur. J. Cancer 27, 954–958 (1991).
Oosterhof, G. O. et al. Strontium(89) chloride versus palliative local field radiotherapy in patients with hormonal escaped prostate cancer: a phase III study of the European Organisation for Research and Treatment of Cancer, Genitourinary Group. Eur. Urol. 44, 519–526 (2003).
Smeland, S. et al. Role of strontium-89 as adjuvant to palliative external beam radiotherapy is questionable: results of a double-blind randomized study. Int. J. Radiat. Oncol. Biol. Phys. 56, 1397–1404 (2003).
Quilty, P. M. et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother. Oncol. 31, 33–40 (1994).
Finlay, I. G., Mason, M. D. & Shelley, M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncol. 6, 392–400 (2005).
Kasalický, J. & Krajská, V. The effect of repeated strontium-89 chloride therapy on bone pain palliation in patients with skeletal cancer metastases. Eur. J. Nucl. Med. 25, 1362–1367 (1998).
Dafermou, A. et al. A multicentre observational study of radionuclide therapy in patients with painful bone metastases of prostate cancer. Eur. J. Nucl. Med. 28, 788–798 (2001).
Bayouth, J. E., Macey, D. J., Kasi, L. P. & Fossella, F. V. Dosimetry and toxicity of samarium-153-EDTMP administered for bone pain due to skeletal metastases. J. Nucl. Med. 35, 63–69 (1994).
Collins, C. et al. Samarium-153-EDTMP in bone metastases of hormone refractory prostate carcinoma: a phase I/II trial. J. Nucl. Med. 34, 1839–1844 (1993).
Turner, J. H., Claringbold, P. G., Hetherington, E. L., Sorby, P. & Martindale, A. A. A phase I study of samarium-153 ethylenediaminetetramethylene phosphonate therapy for disseminated skeletal metastases. J. Clin. Oncol. 7, 1926–1931 (1989).
Serafini, A. N. et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J. Clin. Oncol. 16, 1574–1581 (1998).
Sartor, O. et al. Samarium-153-lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 63, 940–945 (2004).
Resche, I. et al. A dose-controlled study of 153Sm-ethylenediaminetetramethylenephosphonate (EDTMP) in the treatment of patients with painful bone metastases. Eur. J. Cancer 33, 1583–1591 (1997).
El-Amm, J., Freeman, A., Patel, N. & Aragon-Ching, J. B. Bone-targeted therapies in metastatic castration-resistant prostate cancer: evolving paradigms. Prostate Cancer 2013, 210686 (2013).
Lassmann, M. & Nosske, D. Dosimetry of 223Ra-chloride: dose to normal organs and tissues. Eur. J. Nucl. Med. Mol. Imaging 40, 207–212 (2013).
US Food and Drug Administration. Highlights of prescribing information, Xofigo (radium Ra 223 dichloride) Injection, for intravenous use [online], (2013).
Carrasquillo, J. A. et al. Phase I pharmacokinetic and biodistribution study with escalating doses of 223Ra-dichloride in men with castration-resistant metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 40, 1384–1393 (2013).
Nilsson, S. et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin. Cancer Res. 11, 4451–4459 (2005).
Nilsson, S. et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 8, 587–594 (2007).
Nilsson, S. et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur. J. Cancer 48, 678–686 (2012).
Parker, C. C. et al. A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur. Urol. 63, 189–197 (2013).
Hoskin, P. et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 15, 1397–1406 (2014).
European Medicines Agency. Xofigo: EPAR - Product Information [online] (2015).
US National Libraries of Medicine. ClinicalTrials.gov [online], (2015).
US National Libraries of Medicine. ClinicalTrials.gov [online], (2015).
US National Libraries of Medicine. ClinicalTrials.gov [online], (2015).
US National Libraries of Medicine. ClinicalTrials.gov [online], (2015).
Beer, T. M. et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J. Clin. Oncol. 25, 669–674 (2007).
Carducci, M. A. et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer 110, 1959–1966 (2007).
Nelson, J. B. et al. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer 118, 5709–5718 (2012).
Quinn, D. I. et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol. 14, 893–900 (2013).
Fizazi, K. S. et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 31, 1740–1747 (2013).
Saad, F. & Lipton, A. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat. Rev. 36, 177–184 (2010).
Yu, E. Y. et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 15, 7421–7428 (2009).
Yu, E. Y. et al. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology 77, 1166–1171 (2011).
Twardowski, P. W. et al. A phase II trial of dasatinib in patients with metastatic castration-resistant prostate cancer treated previously with chemotherapy. Anticancer Drugs 24, 743–753 (2013).
Araujo, J. C. et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1–2 study. Cancer 118, 63–71 (2012).
Araujo, J. C. et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 14, 1307–1316 (2013).
Smith, D. C. et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J. Clin. Oncol. 31, 412–419 (2013).
Dai, J. et al. Cabozantinib inhibits prostate cancer growth and prevents tumor-induced bone lesions. Clin. Cancer Res. 20, 617–630 (2014).
Exelixis. Exelixis announces results from the COMET-1 phase 3 pivotal trial of cabozantinib in men with metastatic castration-resistant prostate cancer [online], (2014).
US National Libraries of Medicine. ClinicalTrials.gov [online], (2015).
US National Libraries of Medicine. ClinicalTrials.gov [online], (2015).
Mostaghel, E. A., Montgomery, B. & Nelson, P. S. Castration-resistant prostate cancer: targeting androgen metabolic pathways in recurrent disease. Urol. Oncol. 27, 251–257 (2009).
Mantalaris, A. et al. Localization of androgen receptor expression in human bone marrow. J. Pathol. 193, 361–366 (2001).
Niu, Y. et al. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene 29, 3593–3604 (2010).
Reid, A. H., Attard, G., Barrie, E. & de Bono, J. S. CYP17 inhibition as a hormonal strategy for prostate cancer. Nat. Clin. Pract. Urol. 5, 610–620 (2008).
Logothetis, C. J. et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 13, 1210–1217 (2012).
Basch, E. et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 14, 1193–1199 (2013).
Ryan, C. J. et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 16, 152–160 (2015).
El-Amm, J., Patel, N., Freeman, A. & Aragon-Ching, J. B. Metastatic castration-resistant prostate cancer: critical review of enzalutamide. Clin. Med. Insights Oncol. 7, 235–245 (2013).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Loriot, Y. et al. Impact of enzalutamide on skeletal related events (SREs), pain and quality of life (QoL) in the PREVAIL trial [abstract]. Ann. Oncol. 25 (Suppl. 4), 762PD (2014).
Coleman, R. et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit. Rev. Oncol. Hematol. 80, 411–432 (2011).
Lara, P. N. Jr et al. Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J. Natl Cancer Inst. 106, dju013 (2014).
Fitzpatrick, J. M. et al. Optimal management of metastatic castration-resistant prostate cancer: highlights from a European expert consensus panel. Eur. J. Cancer 50, 1617–1627 (2014).
Sweeney, C. et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase III randomized trial [abstract]. J. Clin. Oncol. 32 (Suppl. 18), LBA2 (2014).
Omlin, A., Pezaro, C. & Gillessen Sommer, S. Sequential use of novel therapeutics in advanced prostate cancer following docetaxel chemotherapy. Ther. Adv. Urol. 6, 3–14 (2014).
Hurwitz, M. & Petrylak, D. P. Sequencing of agents for castration-resistant prostate cancer. Oncology (Williston Park) 27, 1144–1149, 1154–1158 (2013).
Smith, M. R. et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379, 39–46 (2012).
Smith, M. R. et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J. Clin. Oncol. 31, 3800–3806 (2013).
James, N. D. et al. Clinical outcomes in patients with castrate-refractory prostate cancer (CRPC) metastatic to bone randomized in the factorial TRAPEZE trial to docetaxel (D) with strontium-89 (Sr89), zoledronic acid (ZA), neither, or both (ISRCTN 12808747) [abstract]. J. Clin. Oncol. 31 (Suppl. 18), LBA5000 (2013).
Morris, M. J. et al. Safety of radium-223 dichloride (Ra-223) with docetaxel (D) in patients with bone metastases from castration-resistant prostate cancer (CRPC): a phase I Prostate Cancer Clinical trials Consortium study [abstract]. J. Clin. Oncol. 31 (Suppl. 15), 5021 (2013).
Saad, F. et al. Impact of concomitant bone-targeted therapies (BTT) on outcomes in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) without prior chemotherapy (ctx) treated with abiraterone acetate (AA) or prednisone (P) [abstract]. J. Clin. Oncol. 31 (Suppl. 15), 5037 (2013).
Araujo, A., Cook, L. M., Lynch, C. C. & Basanta, D. An integrated computational model of the bone microenvironment in bone-metastatic prostate cancer. Cancer Res. 74, 2391–2401 (2014).
Zhao, B., Pritchard, J. R., Lauffenburger, D. A. & Hemann, M. T. Addressing genetic tumor heterogeneity through computationally predictive combination therapy. Cancer Discov. 4, 166–174 (2014).
Acknowledgements
The authors would like to express their gratitude to Dr P. Hoban (Cancer Communications & Consultancy Ltd, Knutsford, UK) for providing writing assistance funded by Bayer Consumer Care AG.
Author information
Authors and Affiliations
Contributions
All authors were involved in researching data for the article, made substantial contributions to discussion of its content and were involved in writing and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.J.B. has received speaker's fees from Amgen and consulting fees from Amgen and Bayer. L.C. has received research funding and speaker's fees from Amgen, Novartis, and Roche. S.C. declares no competing interests.
Supplementary information
Supplementary Table 1
Summary of efficacy data from randomised trials of bone targeting-agents approved in mCRPC (PDF 166 kb)
Supplementary Table 2
Selected studies of Ra-223 in mCRPC with bone metastases (PDF 105 kb)
Supplementary Table 3
Summary of efficacy data of antineoplastic agents approved for patients with CRPC with bone metastases (PDF 160 kb)
Rights and permissions
About this article
Cite this article
Body, JJ., Casimiro, S. & Costa, L. Targeting bone metastases in prostate cancer: improving clinical outcome. Nat Rev Urol 12, 340–356 (2015). https://doi.org/10.1038/nrurol.2015.90
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2015.90
This article is cited by
-
Acetylation of KLF5 maintains EMT and tumorigenicity to cause chemoresistant bone metastasis in prostate cancer
Nature Communications (2021)
-
Endolysosomal ion channel MCOLN2 (Mucolipin-2) promotes prostate cancer progression via IL-1β/NF-κB pathway
British Journal of Cancer (2021)
-
LncRNA SNHG1 and RNA binding protein hnRNPL form a complex and coregulate CDH1 to boost the growth and metastasis of prostate cancer
Cell Death & Disease (2021)
-
Matching-Adjusted Indirect Comparison of Health-Related Quality of Life and Adverse Events of Apalutamide Versus Enzalutamide in Non-Metastatic Castration-Resistant Prostate Cancer
Advances in Therapy (2020)
-
Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer
Cancer Communications (2019)