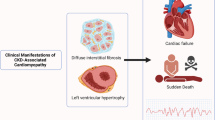
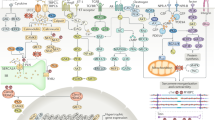
Key Points
-
Heart failure (HF) interacts with kidney disease via numerous pathophysiological pathways in both the acute and chronic setting
-
Mounting data indicate that the complex interplay between the heart and the kidneys involves haemodynamic, (neuro)homonal and cardiovascular disease-associated mechanisms
-
Acceleration of HF or kidney dysfunction is driven by impairment of either the heart or kidneys via mechanisms including induction of inflammation, activation of the cellular immune system, metabolic disorders, anaemia and mineral and bone disorder
-
In an effort to differentiate respective underlying pathologies and to assess acute and/or chronic organ dysfunction over time, five subtypes of cardio-renal syndromes were proposed
-
The absence of a standardized terminology database and the lack of studies specific to cardio-renal syndrome has hampered efforts to develop novel treatments
Abstract
Heart failure (HF) is a major health-care problem and the prognosis of affected patients is poor. HF often coexists with a number of comorbidities of which declining renal function is of particular importance. A loss of glomerular filtration rate, as in acute kidney injury (AKI) or chronic kidney disease (CKD), independently predicts mortality and accelerates the overall progression of cardiovascular disease and HF. Importantly, cardiac and renal diseases interact in a complex bidirectional and interdependent manner in both acute and chronic settings. From a pathophysiological perspective, cardiac and renal diseases share a number of common pathways, including inflammatory and direct, cellular immune-mediated mechanisms; stress-mediated and (neuro)hormonal responses; metabolic and nutritional changes including bone and mineral disorder, altered haemodynamic and acid–base or fluid status; and the development of anaemia. In an effort to better understand the important crosstalk between the two organs, classifications such as the cardio-renal syndromes were developed. This classification might lead to a more precise understanding of the complex interdependent pathophysiology of cardiac and renal diseases. In light of exceptionally high mortality associated with coexisting HF and kidney disease, this Review describes important crosstalk between the heart and kidney, with a focus on HF and kidney disease in the acute and chronic settings. Underlying molecular and cellular pathomechanisms in HF, AKI and CKD are discussed in addition to current and future therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schocken, D. D. et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 117, 2544–2565 (2008).
van Riet, E. E. et al. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 18, 242–252 (2016).
Redfield, M. M. et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289, 194–202 (2003).
Bagshaw, S. M. et al. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol. Dial. Transplant. 25, 1406–1416 (2010).
House, A. A. et al. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol. Dial. Transplant. 25, 1416–1420 (2010).
Segall, L., Nistor, I. & Covic, A. Heart failure in patients with chronic kidney disease: a systematic integrative review. BioMed Res. Int. 2014, 937398 (2014).
Mentz, R. J., O'Connor, C. M. Pathophysiology and clinical evaluation of acute heart failure. Nat. Rev. Cardiol. 13, 28–35 (2016).
Filippatos, G., Farmakis, D. & Parissis, J. Renal dysfunction and heart failure: things are seldom what they seem. Eur. Heart J. 35, 416–418 (2014).
Mehta, R. L. et al. Acute Kidney Injury Network. Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 11, R31 (2007).
Damman, K., Tang, W. H., Testani, J. M. & McMurray, J. J. Terminology and definition of changes renal function in heart failure. Eur. Heart J. 35, 3413–3416 (2014).
Hogg, K., Swedberg, K. & McMurray, J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J. Am. College Cardiol. 43, 317–327 (2004).
McMurray, J. J. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 33, 1787–1847 (2012).
Ezekowitz, J. et al. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J. Am. College Cardiol. 44, 1587–1592 (2004).
Hillege, H. L. et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 113, 671–678 (2006).
McAlister, F. A., Ezekowitz, J., Tonelli, M. & Armstrong, P. W. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 109, 1004–1009 (2004).
McClellan, W. M., Langston, R. D. & Presley, R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J. Am. Soc. Nephrol. 15, 1912–1919 (2004).
van Deursen, V. M. et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur. J. Heart Fail. 16, 103–111 (2014).
Morley, J. E., Anker, S. D. & von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J. Cachexia Sarcopenia Muscle 5, 253–259 (2014).
Adams, K. F. et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am. Heart J. 149, 209–216 (2005).
Tonelli, M. et al. Chronic kidney disease and mortality risk: a systematic review. J. Am. Soc. Nephrol. 17, 2034–2047 (2006).
Sud, M., Tangri, N., Pintilie, M., Levey, A. S. & Naimark, D. Risk of end-stage renal disease and death after cardiovascular events in chronic kidney disease. Circulation 130, 458–465 (2014).
Gansevoort, R. T. et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382, 339–352 (2013).
United States Renal Data System. Chronic kidney disease in the adult NHANES population. USRDS annual report data http://www.usrds.org/2009/pdf/V1_01_09.pdf (2009).
Levey, A. S. et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 80, 17–28 (2011).
Kottgen, A. et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J. Am. Soc. Nephrol. 18, 1307–1315 (2007).
Sud, M., Tangri, N., Pintilie, M., Levey, A. S. & Naimark, D. M. ESRD and death after heart failure in CKD. J. Am. Soc. Nephrol. 26, 715–722 (2015).
Tsuruya, K., Eriguchi, M., Yamada, S., Hirakata, H. & Kitazono, T. Cardio-renal syndrome in end-stage kidney disease. Blood Purif. 40, 337–343 (2015).
Johnson, D. W., Craven, A. M. & Isbel, N. M. Modification of cardiovascular risk in hemodialysis patients: an evidence-based review. Hemodial. Int. 11, 1–14 (2007).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl. J. Med. 351, 1296–1305 (2004).
Foley, R. N., Parfrey, P. S. & Sarnak, M. J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 32 (Suppl. 3), S112–S119 (1998).
Foley, R. N. et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 47, 186–192 (1995).
Soucie, J. M. & McClellan, W. M. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. J. Am. Soc. Nephrol. 7, 2169–2175 (1996).
Harnett, J. D. et al. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int. 47, 884–890 (1995).
Wang, A. Y. et al. Heart failure in long-term peritoneal dialysis patients: a 4-year prospective analysis. Clin. J. Am. Soc. Nephrol. 6, 805–812 (2011).
Basile, D. P., Anderson, M. D. & Sutton, T. A. Pathophysiology of acute kidney injury. Comprehensive Physiol. 2, 1303–1353 (2012).
Berl, T. & Henrich, W. Kidney-heart interactions: epidemiology, pathogenesis, and treatment. Clin. J. Am. Soc. Nephrol. 1, 8–18 (2006).
Chertow, G. M., Burdick, E., Honour, M., Bonventre, J. V. & Bates, D. W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 16, 3365–3370 (2005).
Nash, K., Hafeez, A. & Hou, S. Hospital-acquired renal insufficiency. Am. J. Kidney Dis. 39, 930–936 (2002).
Uchino, S. et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294, 813–818 (2005).
Bagshaw, S. M., George, C., Dinu, I. & Bellomo, R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol. Dial. Transplant. 23, 1203–1210 (2008).
Hsu, C. Y. et al. Community-based incidence of acute renal failure. Kidney Int. 72, 208–212 (2007).
Waikar, S. S., Curhan, G. C., Wald, R., McCarthy, E. P. & Chertow, G. M. Declining mortality in patients with acute renal failure, 1988 to 2002. J. Am. Soc. Nephrol. 17, 1143–1150 (2006).
Kolhe, N. V., Muirhead, A. W., Wilkes, S. R., Fluck, R. J. & Taal, M. W. The epidemiology of hospitalised acute kidney injury not requiring dialysis in England from 1998 to 2013: retrospective analysis of hospital episode statistics. Int. J. Clin. Practice 70, 330–339 (2016).
Martensson, J. & Bellomo, R. Sepsis-induced acute kidney injury. Crit. Care Clin. 31, 649–660 (2015).
Rossaint, J. & Zarbock, A. Acute kidney injury: definition, diagnosis and epidemiology. Minerva Urol. Nefrol. 68, 49–57 (2015).
Damman, K. et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur. Heart J. 35, 455–469 (2014).
Davison, B. A. et al. Worsening heart failure following admission for acute heart failure: a pooled analysis of the PROTECT and RELAX-AHF Studies. JACC Heart Fail. 3, 395–403 (2015).
Grams, M. E. & Rabb, H. The distant organ effects of acute kidney injury. Kidney Int. 81, 942–948 (2012).
Metra, M. et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circul. Heart Fail. 5, 54–62 (2012).
Whitman, I. R., Feldman, H. I. & Deo, R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J. Am. Soc. Nephrol. 23, 1929–1939 (2012).
Ronco, C., Haapio, M., House, A. A., Anavekar, N. & Bellomo, R. Cardio-renal syndrome. J. Am. College Cardiol. 52, 1527–1539 (2008).
McCullough, P. A. et al. Pathophysiology of the cardio-renal syndromes: executive summary from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib. Nephrol. 182, 82–98 (2013).
Hadjiphilippou, S. & Kon, S. P. Cardio-renal syndrome: review of our current understanding. J. R. Soc. Med. 109, 12–17 (2016).
Tsuruya, K. & Eriguchi, M. Cardio-renal syndrome in chronic kidney disease. Curr. Opin. Nephrol. Hypertension 24, 154–162 (2015).
Mall, G., Huther, W., Schneider, J., Lundin, P. & Ritz, E. Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol. Dial. Transplant. 5, 39–44 (1990).
Wali, R. K. et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J. Am. College Cardiol. 45, 1051–1060 (2005).
Bock, J. S. & Gottlieb, S. S. Cardio-renal syndrome: new perspectives. Circulation 121, 2592–2600 (2010).
Waldum, B. & Os, I. The cardio-renal syndrome: what the cardiologist needs to know. Cardiology 126, 175–186 (2013).
Graziani, G. et al. Renal dysfunction in acute congestive heart failure: a common problem for cardiologists and nephrologists. Heart Fail. Rev. 19, 699–708 (2014).
Carlstrom, M., Wilcox, C. S. & Arendshorst, W. J. Renal autoregulation in health and disease. Physiol. Rev. 95, 405–511 (2015).
Ljungman, S., Laragh, J. H. & Cody, R. J. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs 39 (Suppl. 4), 10–21 (1990).
Singh, D. K., Winocour, P. & Farrington, K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat. Clin. Practice Nephrol. 4, 216–226 (2008).
Manotham, K. et al. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int. 65, 871–880 (2004).
Norman, J. T., Clark, I. M. & Garcia, P. L. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 58, 2351–2366 (2000).
Heywood, J. T. et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J. Cardiac Fail. 13, 422–430 (2007).
Nohria, A. et al. Cardio-renal interactions: insights from the ESCAPE trial. J. Am. College Cardiol. 51, 1268–1274 (2008).
Mullens, W. et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. College Cardiol. 53, 589–596 (2009).
Drazner, M. H., Rame, J. E., Stevenson, L. W. & Dries, D. L. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. New Engl. J. Med. 345, 574–581 (2001).
Maeder, M. T., Holst, D. P. & Kaye, D. M. Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J. Cardiac Fail. 14, 824–830 (2008).
Mullens, W. et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J. Am. College Cardiol. 51, 300–306 (2008).
Chen, K. P. et al. Peripheral edema, central venous pressure, and risk of AKI in critical illness. Clin. J. Am. Soc. Nephrol. 11, 602–608 (2016).
Damman, K. et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. College Cardiol. 53, 582–588 (2009).
Guyton, A. C. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol. Rev. 35, 123–129 (1955).
Uemura, K. et al. A novel framework of circulatory equilibrium. Am. J. Physiol. Heart Circulatory Physiol. 286, H2376–2385 (2004).
Magder, S. Point: the classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J. Appl. Physiol. 101, 1523–1525 (2006).
Winton, F. R. The influence of venous pressure on the isolated mammalian kidney. J. Physiol. 72, 49–61 (1931).
Gottschalk, C. W. & Mylle, M. Micropuncture study of pressures in proximal tubules and peritubular capillaries of the rat kidney and their relation to ureteral and renal venous pressures. Am. J. Physiol. 185, 430–439 (1956).
Doty, J. M. et al. Effect of increased renal venous pressure on renal function. J. Trauma 47, 1000–1003 (1999).
Braam, B., Joles, J. A., Danishwar, A. H. & Gaillard, C. A. Cardio-renal syndrome — current understanding and future perspectives. Nat. Rev. Nephrol. 10, 48–55 (2014).
Ronco, C. et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur. Heart J. 31, 703–711 (2010).
Damman, K., Voors, A. A., Navis, G., van Veldhuisen, D. J. & Hillege, H. L. The cardio-renal syndrome in heart failure. Prog. Cardiovasc. Dis. 54, 144–153 (2011).
Afsar, B. et al. Focus on renal congestion in heart failure. Clin. Kidney J. 9, 39–47 (2016).
Erly, B. et al. Hepatorenal syndrome: a review of pathophysiology and current treatment options. Semin. Intervent. Radiol. 32, 445–454 (2015).
Verbrugge, F. H. et al. Abdominal contributions to cardio-renal dysfunction in congestive heart failure. J. Am. College Cardiol. 62, 485–495 (2013).
Volpe, M., Carnovali, M. & Mastromarino, V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin. Sci. 130, 57–77 (2016).
Ruiz-Ortega, M. et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int. Suppl. 82, S12–S22 (2002).
Sharma, U. C. et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110, 3121–3128 (2004).
de Boer, R. A. et al. Galectin-3 in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 15, 1095–1101 (2013).
AbouEzzeddine, O.F. et al. Galectin-3 in heart failure with preserved ejection fraction. A RELAX trial substudy (phosphodiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure). JACC Heart Fail. 3, 245–252 (2015).
Shenker, Y., Sider, R. S., Ostafin, E. A. & Grekin, R. J. Plasma levels of immunoreactive atrial natriuretic factor in healthy subjects and in patients with edema. J. Clin. Invest. 76, 1684–1687 (1985).
Trimarco, B. et al. Blunted sympathetic response to cardiopulmonary receptor unloading in hypertensive patients with left ventricular hypertrophy. A possible compensatory role of atrial natriuretic factor. Circulation 80, 883–892 (1989).
Volpe, M. et al. Carotid baroreceptor unloading decreases plasma atrial natriuretic factor in hypertensive patients. J. Hypertension 4, S519–S522 (1986).
von Haehling, S. et al. Comparison of midregional pro-atrial natriuretic peptide with N-terminal pro-B-type natriuretic peptide in predicting survival in patients with chronic heart failure. J. Am. College Cardiol. 50, 1973–1980 (2007).
Gegenhuber, A. et al. Midregional pro-A-type natriuretic peptide measurements for diagnosis of acute destabilized heart failure in short-of-breath patients: comparison with B-type natriuretic peptide (BNP) and amino-terminal proBNP. Clin. Chem. 52, 827–831 (2006).
Moertl, D. et al. Comparison of midregional pro-atrial and B-type natriuretic peptides in chronic heart failure: influencing factors, detection of left ventricular systolic dysfunction, and prediction of death. J. Am. College Cardiol. 53, 1783–1790 (2009).
Voors, A. A. et al. C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur. Heart J. 30, 1187–1194 (2009).
Farmakis, D., Filippatos, G., Kremastinos, D. T. & Gheorghiade, M. Vasopressin and vasopressin antagonists in heart failure and hyponatremia. Curr. Heart Fail. Rep. 5, 91–96 (2008).
Grassi, G. et al. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 57, 846–851 (2011).
Zoccali, C. et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 105, 1354–1359 (2002).
Dibona, G. F., Jones, S. Y. & Sawin, L. L. Reflex influences on renal nerve activity characteristics in nephrosis and heart failure. J. Am. Soc. Nephrol. 8, 1232–1239 (1997).
DiBona, G. F. & Kopp, U. C. Neural control of renal function. Physiol. Rev. 77, 75–197 (1997).
Ramchandra, R. & Barrett, C. J. Regulation of the renal sympathetic nerves in heart failure. Frontiers Physiol. 6, 238 (2015).
Krum, H. et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373, 1275–1281 (2009).
Schepers, E. et al. Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 2374–2383 (2011).
Bongartz, L. G. et al. Transient nitric oxide reduction induces permanent cardiac systolic dysfunction and worsens kidney damage in rats with chronic kidney disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R815–R823 (2010).
Bongartz, L. G. et al. Subtotal nephrectomy plus coronary ligation leads to more pronounced damage in both organs than either nephrectomy or coronary ligation. Am. J. Physiol. Heart Circulatory Physiol. 302, H845–H854
Voors, A. A. et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the selective adenosine A1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function). J. Am. College Cardiol. 57, 1899–1907 (2011).
Kato, S. et al. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 3, 1526–1533 (2008).
Machowska, A., Carrero, J. J., Lindholm, B. & Stenvinkel, P. Therapeutics targeting persistent inflammation in chronic kidney disease. Translat. Res. 167, 204–213 (2016).
von Haehling, S. et al. Leukocyte redistribution: effects of beta blockers in patients with chronic heart failure. PLoS ONE 4, e6411 (2009).
Stenvinkel, P., Heimburger, O., Lindholm, B., Kaysen, G.A. & Bergstrom, J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol. Dial. Transplant. 15, 953–960 (2000).
Silverberg, D. S., Wexler, D., Blum, M. & Iaina, A. The cardio renal anemia syndrome: correcting anemia in patients with resistant congestive heart failure can improve both cardiac and renal function and reduce hospitalizations. Clin. Nephrol. 60 (Suppl. 1), S93–S102 (2003).
Silverberg, D. S. et al. The effect of correction of anaemia in diabetics and non-diabetics with severe resistant congestive heart failure and chronic renal failure by subcutaneous erythropoietin and intravenous iron. Nephrol. Dial. Transplant. 18, 141–146 (2003).
Kidney disease: improving global outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 113, S1–S130 (2009).
Mangner, N. et al. Skeletal muscle alterations in chronic heart failure: differential effects on quadriceps and diaphragm. J. Cachexia Sarcopenia Muscle 6, 381–390 (2015).
Josiak, K., Jankowska, E. A., Piepoli, M. F., Banasiak, W. & Ponikowski, P. Skeletal myopathy in patients with chronic heart failure: significance of anabolic-androgenic hormones. J. Cachexia, Sarcopenia Muscle 5, 287–296 (2014).
von Haehling, S., Schefold, J. C., Lainscak, M., Doehner, W. & Anker, S. D. Inflammatory biomarkers in heart failure revisited: much more than innocent bystanders. Heart Fail. Clin. 5, 549–560 (2009).
Colombo, P. C. et al. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardio-renal syndrome. Heart Fail. Rev. 17, 177–190 (2012).
Stenvinkel, P. & Larsson, T. E. Chronic kidney disease. a clinical model of premature aging. Am. J. Kidney Diseases 62, 339–351 (2013).
Kooman, J. P., Kotanko, P., Schols, A. M., Shiels, P. G. & Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 10, 732–742 (2014).
Schefold, J. P. et al. Increased Indoleamine 2,3-Dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol. Dial. Transplant. 24, 1901–1908 (2009).
Kitai, T., Kirsop, J. & Tang, W. H. Exploring the Microbiome in Heart Failure. Curr. Heart Fail. Rep. 13, 103–109 (2016).
Barreto, D. V. et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 77, 550–556 (2010).
Ismahil, M. A. et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circul. Res. 114, 266–282 (2014).
Hoffmann, J. et al. Myocardial ischemia and reperfusion leads to transient CD8 immune deficiency and accelerated immunosenescence in CMV-seropositive patients. Circul. Res. 116, 87–98 (2015).
von Haehling, S. The wasting continuum in heart failure: from sarcopenia to cachexia. Proc. Nutr. Soc. 74, 367–377 (2015).
Ebner, N., Elsner, S., Springer, J. & von Haehling, S. Molecular mechanisms and treatment targets of muscle wasting and cachexia in heart failure: an overview. Curr. Opin. Supportive Palliative Care 8, 15–24 (2014).
Ebner, N. et al. Mechanism and novel therapeutic approaches to wasting in chronic disease. Maturitas 75, 199–206 (2013).
von Haehling, S., Lainscak, M., Springer, J. & Anker, S. D. Cardiac cachexia: a systematic overview. Pharmacol. Ther. 121, 227–252 (2009).
von Haehling, S., Steinbeck, L., Doehner, W., Springer, J. & Anker, S. D. Muscle wasting in heart failure: an overview. Int. J. Biochem. Cell Biol. 45, 2257–2265 (2013).
Pecoits-Filho, R., Lindholm, B. & Stenvinkel, P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome — the heart of the matter. Nephrol. Dial. Transpl. 17 (Suppl. 11), 28–31 (2002).
Griendling, K. K., Minieri, C. A., Ollerenshaw, J. D. & Alexander, R. W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circul. Res. 74, 1141–1148 (1994).
Heymes, C. et al. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. College Cardiol. 41, 2164–2171 (2003).
Vaziri, N. D., Dicus, M., Ho, N. D., Boroujerdi-Rad, L. & Sindhu, R. K. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 63, 179–185 (2003).
Munzel, T., Gori, T. & Keaney, J. F. Jr., Maack, C. & Daiber, A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J. 36, 2555–2564 (2015).
Ratcliffe, P. J. From erythropoietin to oxygen: hypoxia-inducible factor hydroxylases and the hypoxia signal pathway. Blood Purif. 20, 445–450 (2002).
Pschowski, R. et al. Effects of dialysis modality on blood loss, bleeding complications and transfusion requirements in critically ill patients with dialysis-dependent acute renal failure. Anaesth. Intensive Care 43, 764–770 (2015).
Lin, C. L. et al. Increased blood loss from access cannulation site during hemodialysis is associated with anemia and arteriovenous graft use. Ther. Apher. Dial. 18, 51–56 (2014).
Groenveld, H. F. et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J. Am. College Cardiol. 52, 818–827 (2008).
Maggioni, A. P. et al. Anemia in patients with heart failure: prevalence and prognostic role in a controlled trial and in clinical practice. J. Cardiac Fail. 11, 91–98 (2005).
Kawashiro, N. et al. Clinical characteristics and outcome of hospitalized patients with congestive heart failure: results of the HIJC-HF registry. Circul. J. 72, 2015–2020 (2008).
Young, J. B. et al. Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE-HF registry). Am. J. Cardiol. 101, 223–230 (2008).
von Haehling, S. et al. Anaemia is an independent predictor of death in patients hospitalized for acute heart failure. Clin. Res. Cardiol. 99, 107–113 (2010).
von Haehling, S. et al. Anaemia among patients with heart failure and preserved or reduced ejection fraction: results from the SENIORS study. Eur. J. Heart Fail. 13, 656–663 (2011).
van der Meer, P., Groenveld, H. F. & Januzzi, J. L. Jr., van Veldhuisen, D. J. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart 95, 1309–1314 (2009).
Anker, S. D. et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. New Engl. J. Med. 361, 2436–2448 (2009).
Ponikowski, P. et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur. Heart J. 36, 657–668 (2015).
von Haehling, S., Jankowska, E. A., van Veldhuisen, D. J., Ponikowski, P. & Anker, S. D. Iron deficiency and cardiovascular disease. Nat. Rev. Cardiol. 12, 659–669 (2015).
Levin, A. et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am. J. Kidney Dis. 34, 125–134 (1999).
Calvillo, L. et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc. Natl Acad. Sci. USA 100, 4802–4806 (2003).
Charytan, D. M., Fishbane, S., Malyszko, J., McCullough, P. A. & Goldsmith, D. Cardio-renal syndrome and the role of the bone-mineral axis and anemia. Am. J. Kidney Dis. 66, 196–205 (2015).
Kovesdy, C. P. & Quarles, L. D. The role of fibroblast growth factor-23 in cardio-renal syndrome. Nephron Clin. Pract. 123, 194–201 (2013).
Achinger, S. G. & Ayus, J. C. Left ventricular hypertrophy: is hyperphosphatemia among dialysis patients a risk factor? J. Am. Soc. Nephrol. 17 S255–S261 (2006).
Fujii, H., Kim, J. I., Abe, T., Umezu, M. & Fukagawa, M. Relationship between parathyroid hormone and cardiac abnormalities in chronic dialysis patients. Internal Med. 46, 1507–1512 (2007).
Scialla, J. J. & Wolf, M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat. Rev. Nephrol. 10, 268–278 (2014).
Rozentryt, P. et al. Higher serum phosphorus is associated with catabolic/anabolic imbalance in heart failure. J. Cachexia Sarcopenia Muscle 6, 325–334 (2015).
Moe, S. M. et al. Cinacalcet, Fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Circulation 132, 27–39 (2015).
Wang, A. Y. et al. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. Am. J. Clin. Nutr. 87, 1631–1638 (2008).
Panizo, S. et al. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol. Dial. Transplant. 28, 2735–2744 (2013).
de Zeeuw, D. et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376, 1543–1551 (2010).
Thadhani, R. et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 307, 674–684 (2012).
Garg, N. et al. Cardiac resynchronization therapy in CKD: a systematic review. Clin. J. Am. Soc. Nephrol. 8, 1293–1303 (2013).
Kidney disease: improving global outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. 2, 9–335 (2012).
Gastelurrutia, P. et al. Body mass index, body fat, and nutritional status of patients with heart failure: The PLICA study. Clin. Nutr. 34, 1233–1238 (2015).
Khalid, U. et al. Pre-morbid body mass index and mortality after incident heart failure: the ARIC study. J. Am. College Cardiol. 64, 2743–2749 (2014).
Rozentryt, P. et al. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J. Cachexia Sarcopenia Muscle 1, 35–42 (2010).
Witte, K. K. et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur. Heart J. 26, 2238–2244 (2005).
Valentova, M. et al. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur. Heart J. 37, 1684–1691 (2016).
[No authors listed] Retraction. Low sodium versus normal sodium diets in systolic heart failure: systematic review and meta-analysis. Heart 99, 820 (2013).
DiNicolantonio, J. J., Di Pasquale, P., Taylor, R. S. & Hackam, D. G. Low sodium versus normal sodium diets in systolic heart failure: systematic review and meta-analysis. Heart http://dx.doi.org/10.1136/heartjnl-2012-302337 (2013).
Kidney disease: improving global outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2, 337–414 (2012).
McMurray, J. J. et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. New Engl. J. Med. 371, 993–1004 (2014).
Eshaghian, S., Horwich, T. B. & Fonarow, G. C. Relation of loop diuretic dose to mortality in advanced heart failure. Am. J. Cardiol. 97, 1759–1764 (2006).
ter Maaten, J. M. et al. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat. Rev. Cardiol. 12, 184–192 (2015).
Costanzo, M. R., Fonarow, G. C. & Filippatos, G. S. Ultrafiltration in heart failure with cardio-renal syndrome. New Engl. J. Med. 368, 1158–1159 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT00577135?term=NCT00577135&rank=1 (2016).
US National Library of Medicine. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT01921829?term=NCT01921829&rank=1 (2016).
Lala, A. et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from diuretic optimization strategy evaluation in acute decompensated heart failure (DOSE-AHF) and cardio-renal rescue study in acute decompensated heart failure (CARESS-HF). Circul. Heart Fail. 8, 741–748 (2015).
Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128, 1810–1852 (2013).
Lins, R. L. et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol. Dial. Transplant. 24, 512–518 (2009).
Schefold, J.C. et al. The effect of continuous versus intermittent renal replacement therapy on the outcome of critically ill patients with acute renal failure (CONVINT): a prospective randomized controlled trial. Crit. Care 18, R11 (2014).
Vinsonneau, C. et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet 368, 379–385 (2006).
Zarbock, A. et al. Randomized Clinical Trial. JAMA 20, 2190–2199 (2016).
Seabra, V. F. et al. Timing of renal replacement therapy initiation in acute renal failure: a meta-analysis. Am. J. Kidney Dis. 52, 272–284 (2008).
Kellum, J. A. & Ronco, C. Dialysis: results of RENAL — what is the optimal CRRT target dose? Nat. Rev. Nephrol. 6, 191–192 (2010).
Kidney disease: improving global outcomes (KDIGO). KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2, 1–138 (2012).
Lin, T. E., Adams, K. F. Jr. & Patterson, J.H. Potential roles of vaptans in heart failure: experience from clinical trials and considerations for optimizing therapy in target patients. Heart Fail. Clin. 10, 607–620 (2014).
Lehrich, R. W., Ortiz-Melo, D. I., Patel, M. B. & Greenberg, A. Role of vaptans in the management of hyponatremia. Am. J. Kidney Dis. 62, 364–376 (2013).
Konstam, M. A. et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 297, 1319–1331 (2007).
Morooka, H. et al. Chronic administration of oral vasopressin type 2 receptor antagonist tolvaptan exerts both myocardial and renal protective effects in rats with hypertensive heart failure. Circul. Heart Fail. 5, 484–492 (2012).
Cannella, G. et al. Prolonged therapy with ACE inhibitors induces a regression of left ventricular hypertrophy of dialyzed uremic patients independently from hypotensive effects. Am. J. Kidney Dis. 30, 659–664 (1997).
O'Connor, C. M. et al. Effect of nesiritide in patients with acute decompensated heart failure. New Engl. J. Med. 365, 32–43 (2011).
van Deursen, V. M. et al. Nesiritide, renal function, and associated outcomes during hospitalization for acute decompensated heart failure: results from the Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure (ASCEND-HF). Circulation 130, 958–965 (2014).
Singh, A., Laribi, S., Teerlink, J. R. & Mebazaa, A. Agents with vasodilator properties in acute heart failure. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehv755 (2013).
Teerlink, J. R. et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 381, 29–39 (2013).
Metra, M. et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J. Am. College Cardiol. 61, 196–206 (2013).
Nimmo, A. J., Than, N., Orchard, C. H. & Whitaker, E. M. The effect of acidosis on β-adrenergic receptors in ferret cardiac muscle. Exp. Physiol. 78, 95–103 (1993).
Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 27, 2129–200 (2016).
Author information
Authors and Affiliations
Contributions
J.C.S. and S.v.H. wrote the article. All authors contributed to discussion and researching of the content and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The Department of Intensive Care Medicine, Bern University Hospital, Switzerland (J.C.S.) has received research and development and/or consulting contracts from Orion Corporation, Abbott Nutrition International, B. Braun Medical AG, CSEM SA, Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, Edwards Lifesciences SA, and Nestlé, for which no personal financial gain was received; educational grants from Fresenius Kabi; GSK; MSD; Lilly; Baxter; Astellas; AstraZeneca; B. Braun Medical AG, CSL Behring, Maquet, Novartis, Covidien, Nycomed, Pierre Fabre Pharma (Roba Pharma); Pfizer, Orion Pharma; J.C.S. has received research funding, (travel) grants, or speaker fees from Bayer AG/Schering AG, Eli Lilly, Anagnostics Bionanalysis GmbH/ Cube DX GesmbH, and Nestlé. G.F. is a member of committees of heart failure trials and registries sponsored by Novartis, Servier, Cardiorentis, Medtronic and Vifor. G.H. has consulted for Servier, Impulse Dynamics, Novartis, CircuLite, and DC Devices and received honoraria from CVRx, Impulse Dynamics, AstraZeneca, Bayer, and Orion. S.D.A. has consulted or received honoraria from Vifor, Novartis, Cardiorentis, Brahms, Bayer, Relypsa, ZS Pharma and Stealth Peptides. S.v.H. has received consultant honoraria, travel support, and/or speaker's fees from Vifor, Thermo Fisher Scientific, Respicardia, Sorin, Novartis, Chugai Pharma, AstraZeneca, Pfizer, Professional Dietetics and Solartium Dietetics.
Glossary
- Left ventricular ejection fraction
-
The fraction of left intra-ventricular volume that is pumped from the left ventricle per contraction or heartbeat.
- Renin–angiotensin–aldosterone system
-
A complex hormone system and a key regulator of salt and water homeostasis in humans. The renin–angiotensin–aldosterone system is considered to be one of the key blood pressure regulating hormonal systems. Activation of this system in heart failure and chronic kidney disease makes it particularly important in cardio-renal syndrome.
- Forward failure
-
Term often used by physicians involved in the care of patients with acute heart failure to describe a clinical situation in which left ventricular output is substantially reduced leading to insufficient end-organ and/or peripheral perfusion and/or pulmonary oedema.
- Cardiac index
-
Cardiac index (units: l per min per m2) is a global index of heart function and a quotient of cardiac output and body surface area. This index is important for the monitoring of heart function in critically ill patients in intensive care units.
- Renal congestion
-
Central venous pressure is typically increased in heart failure and acts as the back pressure to venous return, resulting in diminished efferent renal blood flow and renal venous hypertension. Renal congestion is considered to be the result of right ventricular failure, (neuro)hormonal and sympathetic mechanisms resulting in hypervolaemia, inflammation, and reduced glomerular filtration rate.
- Preload
-
The effective end-diastolic volume that stretches the ventricles of the heart before contraction. Ventricular end diastolic volume and/or pressure is used for assessment of preload; atrial pressure might serve as a surrogate marker.
- Starling curve
-
Cardiac function curve showing the graphical relationship between cardiac output or venous return (y-axis) and end diastolic volume or right atrial pressure (x-axis). Frank Starling's law indicates that cardiac output increases in response to increased end diastolic volume (that is, filling) up to a certain maximum (given that all other influencing factors remain constant).
- Increased venous return
-
Increased venous return can almost always be observed in heart failure and results from early activation of key compensatory mechanisms, including neurohormonal responses and activation of the sympathetic nervous system, resulting in increased circulating volume.
- Obesity paradox
-
Epidemiological data show that obese patients with chronic diseases such as heart failure, coronary artery disease or chronic kidney disease requiring dialysis can have higher survival rates compared to those of non-obese individuals.
- Orthodema
-
Key clinical symptoms at rest for congestion include orthopnoea and peripheral oedema. An orthodema congestion score based on these symptoms was previously used to grade congestion in clinical trials.
- Aquaresis
-
Excretion of (free) water without loss of electrolytes via the renal system. Aquaresis is of particular interested in dilutional hyponatraemia. Vaptans (also referred to as aquaretics) are a new class of drugs used to promote aquaresis.
Rights and permissions
About this article
Cite this article
Schefold, J., Filippatos, G., Hasenfuss, G. et al. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol 12, 610–623 (2016). https://doi.org/10.1038/nrneph.2016.113
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.113
This article is cited by
-
Relationship between stress hyperglycemia ratio and acute kidney injury in patients with congestive heart failure
Cardiovascular Diabetology (2024)
-
Sodium glucose cotransporter 2 inhibitor as a promising therapy for congestive kidney injury
Hypertension Research (2024)
-
Evolutions in Combined Heart-Kidney Transplant
Current Heart Failure Reports (2024)
-
Predictors of Kidney Function Outcomes and Their Relation to SGLT2 Inhibitor Dapagliflozin in Patients with Type 2 Diabetes Mellitus Who Had Chronic Heart Failure
Advances in Therapy (2024)
-
The association between proton pump inhibitor use and risk of post-hospitalization acute kidney injury: a multicenter prospective matched cohort study
BMC Nephrology (2023)