Key Points
-
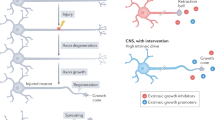
Damage to the adult CNS leads to persistent deficits owing to the inability of CNS axons to regenerate after injury. This regeneration failure is attributable to the reduced intrinsic growth ability of mature neurons and extrinsic inhibitory influences from the glial environment, such as inhibitory molecules in CNS myelin and chondroitin sulphate proteoglycans (CSPGs) from the glial scar.
-
Many myelin-associated inhibitors have been identified using in vitro assays, including Nogo, myelin-associated glycoprotein (MAG) and oligodendrocyte myelin glycoprotein (OMgp). Repulsive guidance cues that are important during development, such as ephrin B3 and semaphorin 4D, might also persist in the adult and limit axon growth.
-
CSPGs expressed by reactive astrocytes can inhibit axon regeneration through their protein core or glycosaminoglycan moieties. Although both CNS myelin and CSPGs are likely to contribute to regeneration failure, their relative importance remains uncertain.
-
Although receptor mechanisms for CSPGs are not known, most myelin inhibitors signal through a common receptor complex that consists of the Nogo-66 receptor (NgR) and its co-receptors p75 or TROY and LINGO1. Recent evidence from genetic deletion studies, however, suggests that there are also NgR-independent signalling pathways.
-
Common intracellular mechanisms probably mediate both CNS myelin and CSPG-based inhibition. The best-characterized pathway involves the small GTPase RhoA and its effector Rho-associated kinase (ROCK), which can regulate the actin cytoskeleton. Calcium-related signals, including protein kinase C and epidermal growth factor (EGFR), might also be involved in these inhibitory pathways.
-
Many in vivo studies have targeted these inhibitory ligands, receptors and downstream components to promote regeneration after spinal cord injury. Whereas some pharmacological and dominant-negative approaches have shown promise, most knockout studies have met with limited success. These results demonstrate the complexity and cross-compensation between the different inhibitory influences, and the potential existence of as yet unidentified mechanisms.
-
Recent reports are revealing intriguing parallels between the mechanisms that prevent axon repair after CNS injury, and those that limit experience-dependent plasticity. Even in the absence of long-distance axon regeneration, recovery from CNS injuries might benefit from local sprouting and structural plasticity similar to the way in which sensory experience fine-tunes neural circuits during the critical period.
-
Alleviating glial inhibition might not only promote the regrowth of damaged axons, but might also enhance recovery through local compensatory sprouting. Combinatorial approaches that target multiple inhibitory pathways and promote the intrinsic growth ability of neurons might be necessary to achieve significant long-distance regeneration.
Abstract
Damage to the adult CNS often leads to persistent deficits due to the inability of mature axons to regenerate after injury. Mounting evidence suggests that the glial environment of the adult CNS, which includes inhibitory molecules in CNS myelin as well as proteoglycans associated with astroglial scarring, might present a major hurdle for successful axon regeneration. Here, we evaluate the molecular basis of these inhibitory influences and their contributions to the limitation of long-distance axon repair and other types of structural plasticity. Greater insight into glial inhibition is crucial for developing therapies to promote functional recovery after neural injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zito, K. & Svoboda, K. Activity-dependent synaptogenesis in the adult mammalian cortex. Neuron 35, 1015–1017 (2002).
Zuo, Y., Yang, G., Kwon, E. & Gan, W. B. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature 436, 261–265 (2005).
Edgerton, V. R., Tillakaratne, N. J., Bigbee, A. J., de Leon, R. D. & Roy, R. R. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 27, 145–167 (2004).
Ramón y Cajal, S. Degeneration and Regeneration of the Nervous System, (Oxford Univ. Press, London, 1928).
Tom, V. J., Steinmetz, M. P., Miller, J. H., Doller, C. M. & Silver, J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 24, 6531–6539 (2004). An interesting study analysing the morphological changes that constitute the dystrophic growth cones of lesioned axons in the hostile CNS environment.
David, S. & Aguayo, A. J. Axonal elongation into peripheral nervous system 'bridges' after central nervous system injury in adult rats. Science 214, 931–933 (1981). A seminal paper that attributes axon regeneration failure to the non-permissive CNS environment by showing that some lesioned axons can regrow through a transplanted peripheral nerve graft.
Filbin, M. T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nature Rev. Neurosci. 4, 703–713 (2003).
He, Z. & Koprivica, V. The Nogo signaling pathway for regeneration block. Annu. Rev. Neurosci. 27, 341–368 (2004).
Yiu, G. & He, Z. Signaling mechanisms of the myelin inhibitors of axon regeneration. Curr. Opin. Neurobiol. 13, 545–551 (2003).
Silver, J. & Miller, J. H. Regeneration beyond the glial scar. Nature Rev. Neurosci. 5, 146–156 (2004).
Schwab, M. E. & Thoenen, H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J. Neurosci. 5, 2415–2423 (1985).
Caroni, P. & Schwab, M. E. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron 1, 85–96 (1988).
Schnell, L. & Schwab, M. E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 343, 269–272 (1990).
Prinjha, R. et al. Inhibitor of neurite outgrowth in humans. Nature 403, 383–284 (2000).
Chen, M. S. et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403, 434–439 (2000).
GrandPre, T., Nakamura, F., Vartanian, T. & Strittmatter, S. M. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403, 439–444 (2000).
Huber, A. B., Weinmann, O., Brosamle, C., Oertle, T. & Schwab, M. E. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 22, 3553–3567 (2002).
Oertle, T. et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J. Neurosci. 23, 5393–5406 (2003).
Fournier, A. E., GrandPre, T. & Strittmatter, S. M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409, 341–346 (2001).
Voeltz, G. K., Prinz, W. A., Shibata, Y., Rist, J. M. & Rapoport, T. A. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124, 573–586 (2006).
Trajkovic, K. et al. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J. Cell Biol. 172, 937–948 (2006).
Mukhopadhyay, G., Doherty, P., Walsh, F. S., Crocker, P. R. & Filbin, M. T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13, 757–767 (1994).
McKerracher, L. et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13, 805–811 (1994).
Wang, K. C. et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417, 941–944 (2002).
Moreau-Fauvarque, C. et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 23, 9229–9239 (2003).
Benson, M. D. et al. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc. Natl Acad. Sci. USA 102, 10694–10699 (2005).
Lai, C., Watson, J. B., Bloom, F. E., Sutcliffe, J. G. & Milner, R. J. Neural protein 1B236/myelin-associated glycoprotein (MAG) defines a subgroup of the immunoglobulin superfamily. Immunol. Rev. 100, 129–151 (1987).
Salzer, J. L., Holmes, W. P. & Colman, D. R. The amino acid sequences of the myelin-associated glycoproteins: homology to the immunoglobulin gene superfamily. J. Cell Biol. 104, 957–965 (1987).
Tang, S., Qiu, J., Nikulina, E. & Filbin, M. T. Soluble myelin-associated glycoprotein released from damaged white matter inhibits axonal regeneration. Mol. Cell. Neurosci. 18, 259–269 (2001).
Schachner, M. & Bartsch, U. Multiple functions of the myelin-associated glycoprotein MAG (siglec-4a) in formation and maintenance of myelin. Glia 29, 154–165 (2000).
Johnson, P. W. et al. Recombinant myelin-associated glycoprotein confers neural adhesion and neurite outgrowth function. Neuron 3, 377–385 (1989).
DeBellard, M. E., Tang, S., Mukhopadhyay, G., Shen, Y. J. & Filbin, M. T. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol. Cell. Neurosci. 7, 89–101 (1996).
Turnley, A. M. & Bartlett, P. F. MAG and MOG enhance neurite outgrowth of embryonic mouse spinal cord neurons. Neuroreport 9, 1987–1990 (1998).
Mikol, D. D., Gulcher, J. R. & Stefansson, K. The oligodendrocyte-myelin glycoprotein belongs to a distinct family of proteins and contains the HNK-1 carbohydrate. J. Cell Biol. 110, 471–479 (1990).
Huang, J. K. et al. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science 310, 1813–1817 (2005). A unique study that provides a spatial explanation for how the myelin-associated inhibitor OMgp can limit collateral sprouting at nodes of Ranvier.
Kullander, K. et al. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 15, 877–888 (2001).
Kim, J. E., Li, S., GrandPre, T., Qiu, D. & Strittmatter, S. M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 38, 187–199 (2003).
Simonen, M. et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38, 201–211 (2003).
Zheng, B. et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 38, 213–224 (2003).
Rudge, J. S. & Silver, J. Inhibition of neurite outgrowth on astroglial scars in vitro. J. Neurosci. 10, 3594–3603 (1990).
Faulkner, J. R. et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155 (2004).
McKeon, R. J., Schreiber, R. C., Rudge, J. S. & Silver, J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 11, 3398–3411 (1991).
Morgenstern, D. A., Asher, R. A. & Fawcett, J. W. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 137, 313–332 (2002).
Snow, D. M., Steindler, D. A. & Silver, J. Molecular and cellular characterization of the glial roof plate of the spinal cord and optic tectum: a possible role for a proteoglycan in the development of an axon barrier. Dev. Biol. 138, 359–376 (1990).
Pindzola, R. R., Doller, C. & Silver, J. Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions. Dev. Biol. 156, 34–48 (1993).
Carulli, D., Laabs, T., Geller, H. M. & Fawcett, J. W. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr. Opin. Neurobiol. 15, 116–120 (2005).
Ughrin, Y. M., Chen, Z. J. & Levine, J. M. Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J. Neurosci. 23, 175–186 (2003).
Bovolenta, P. & Fernaud-Espinosa, I. Nervous system proteoglycans as modulators of neurite outgrowth. Prog. Neurobiol. 61, 113–132 (2000).
Cole, G. J., Loewy, A. & Glaser, L. Neuronal cell–cell adhesion depends on interactions of N-CAM with heparin-like molecules. Nature 320, 445–447 (1986).
Grumet, M., Flaccus, A. & Margolis, R. U. Functional characterization of chondroitin sulfate proteoglycans of brain: interactions with neurons and neural cell adhesion molecules. J. Cell Biol. 120, 815–824 (1993).
Friedlander, D. R. et al. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J. Cell Biol. 125, 669–680 (1994).
McKeon, R. J., Hoke, A. & Silver, J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp. Neurol. 136, 32–43 (1995).
Yang, Z. et al. NG2 glial cells provide a favorable substrate for growing axons. J. Neurosci. 26, 3829–3839 (2006).
Dou, C. L. & Levine, J. M. Identification of a neuronal cell surface receptor for a growth inhibitory chondroitin sulfate proteoglycan (NG2). J. Neurochem. 68, 1021–1030 (1997).
Monnier, P. P., Sierra, A., Schwab, J. M., Henke-Fahle, S. & Mueller, B. K. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol. Cell. Neurosci. 22, 319–330 (2003).
Ramer, M. S., Duraisingam, I., Priestley, J. V. & McMahon, S. B. Two-tiered inhibition of axon regeneration at the dorsal root entry zone. J. Neurosci. 21, 2651–2660 (2001).
Davies, S. J. et al. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390, 680–683 (1997).
Davies, S. J., Goucher, D. R., Doller, C. & Silver, J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 19, 5810–5822 (1999).
Tang, X., Davies, J. E. & Davies, S. J. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J. Neurosci. Res. 71, 427–444 (2003).
Kantor, D. B. et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 44, 961–975 (2004).
Domeniconi, M. et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron 35, 283–990 (2002).
Liu, B. P., Fournier, A., GrandPre, T. & Strittmatter, S. M. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science 297, 1190–1193 (2002).
Yamashita, T., Higuchi, H. & Tohyama, M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol. 157, 565–570 (2002).
Wang, K. C., Kim, J. A., Sivasankaran, R., Segal, R. & He, Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420, 74–78 (2002).
Wong, S. T. et al. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nature Neurosci. 5, 1302–1308 (2002).
Domeniconi, M. et al. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron 46, 849–855 (2005).
Park, J. B. et al. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron 45, 345–351 (2005).
Shao, Z. et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron 45, 353–359 (2005).
Mi, S. et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nature Neurosci. 7, 221–228 (2004).
Fischer, D., He, Z. & Benowitz, L. I. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J. Neurosci. 24, 1646–1651 (2004).
Kim, J. E., Liu, B. P., Park, J. H. & Strittmatter, S. M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 44, 439–451 (2004). A critical paper demonstrating that NgR-deficient mice show enhanced regeneration from raphespinal and rubrospinal tract fibres, but not CST fibres, suggesting that the contribution by NgR to regeneration failure might be different in different nerve fibre tracts.
Zheng, B. et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc. Natl Acad. Sci. USA 102, 1205–1210 (2005). An important study showing that genetic deletion of NgR cannot overcome myelin inhibition or promote CST regeneration, supporting the presence of NgR-independent mechanisms mediating myelin inhibition and regeneration failure.
Lauren, J., Airaksinen, M. S., Saarma, M. & Timmusk, T. Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Mol. Cell. Neurosci. 24, 581–594 (2003).
Pignot, V. et al. Characterization of two novel proteins, NgRH1 and NgRH2, structurally and biochemically homologous to the Nogo-66 receptor. J. Neurochem. 85, 717–728 (2003).
Barton, W. A. et al. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 22, 3291–3302 (2003).
Venkatesh, K. et al. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J. Neurosci. 25, 808–822 (2005).
Niederost, B., Oertle, T., Fritsche, J., McKinney, R. A. & Bandtlow, C. E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 22, 10368–10376 (2002).
Vinson, M. et al. Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition. J. Biol. Chem. 276, 20280–20285 (2001).
Vyas, A. A., Blixt, O., Paulson, J. C. & Schnaar, R. L. Potent glycan inhibitors of myelin-associated glycoprotein enhance axon outgrowth in vitro. J. Biol. Chem. 280, 16305–16310 (2005).
Maekawa, M. et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285, 895–898 (1999).
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Winton, M. J., Dubreuil, C. I., Lasko, D., Leclerc, N. & McKerracher, L. Characterization of new cell permeable C3-like proteins that inactivate Rho and stimulate neurite outgrowth on inhibitory substrates. J. Biol. Chem. 277, 32820–32829 (2002).
Yamashita, T. & Tohyama, M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nature Neurosci. 6, 461–467 (2003).
Dergham, P. et al. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 22, 6570–6577 (2002).
Fournier, A. E., Takizawa, B. T. & Strittmatter, S. M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 23, 1416–1423 (2003).
Lehmann, M. et al. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 19, 7537–7547 (1999).
Hsieh, S. H., Ferraro, G. B. & Fournier, A. E. Myelin-associated inhibitors regulate cofilin phosphorylation and neuronal inhibition through LIM kinase and Slingshot phosphatase. J. Neurosci. 26, 1006–1015 (2006).
Sivasankaran, R. et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nature Neurosci. 7, 261–268 (2004).
Hasegawa, Y. et al. Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J. Neurosci. 24, 6826–6832 (2004).
Koprivica, V. et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 310, 106–110 (2005).
Gomez, T. M. & Spitzer, N. C. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature 397, 350–355 (1999).
Bandtlow, C. E., Schmidt, M. F., Hassinger, T. D., Schwab, M. E. & Kater, S. B. Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science 259, 80–83 (1993).
Snow, D. M., Atkinson, P. B., Hassinger, T. D., Letourneau, P. C. & Kater, S. B. Chondroitin sulfate proteoglycan elevates cytoplasmic calcium in DRG neurons. Dev. Biol. 166, 87–100 (1994).
Brosamle, C., Huber, A. B., Fiedler, M., Skerra, A. & Schwab, M. E. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J. Neurosci. 20, 8061–8068 (2000).
Bartsch, U. et al. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron 15, 1375–1381 (1995).
GrandPre, T., Li, S. & Strittmatter, S. M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 417, 547–551 (2002).
Li, S. & Strittmatter, S. M. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J. Neurosci. 23, 4219–4227 (2003).
Li, S., Kim, J. E., Budel, S., Hampton, T. G. & Strittmatter, S. M. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol. Cell. Neurosci. 29, 26–39 (2005).
Li, S. et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 24, 10511–10520 (2004).
Walsh, G. S., Krol, K. M., Crutcher, K. A. & Kawaja, M. D. Enhanced neurotrophin-induced axon growth in myelinated portions of the CNS in mice lacking the p75 neurotrophin receptor. J. Neurosci. 19, 4155–4168 (1999).
Hannila, S. S. & Kawaja, M. D. Nerve growth factor-induced growth of sympathetic axons into the optic tract of mature mice is enhanced by an absence of p75NTR expression. J. Neurobiol. 39, 51–66 (1999).
Song, X. Y., Zhong, J. H., Wang, X. & Zhou, X. F. Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J. Neurosci. 24, 542–546 (2004).
Menet, V., Prieto, M., Privat, A. & Gimenez y Ribotta, M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc. Natl Acad. Sci. USA 100, 8999–9004 (2003).
Bradbury, E. J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002). A pioneering study describing the use of ChABC to neutralize glial scar-based inhibition as a therapeutic means to promote recovery after CNS injury. This paper spawned many subsequent studies using similar techniques in other injury paradigms.
Moon, L. D., Asher, R. A., Rhodes, K. E. & Fawcett, J. W. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nature Neurosci. 4, 465–466 (2001).
Goldshmit, Y., Galea, M. P., Wise, G., Bartlett, P. F. & Turnley, A. M. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J. Neurosci. 24, 10064–10073 (2004).
Steward, O., Zheng, B. & Tessier-Lavigne, M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J. Comp. Neurol. 459, 1–8 (2003).
Lee, J. K., Kim, J. E., Sivula, M. & Strittmatter, S. M. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 24, 6209–6217 (2004). An interesting paper describing the potential application of blocking inhibitory influences to promote local sprouting and plasticity to enhance stroke recovery.
Papadopoulos, C. M. et al. Dendritic plasticity in the adult rat following middle cerebral artery occlusion and Nogo-a neutralization. Cereb. Cortex 16, 529–536 (2006).
Markus, T. M. et al. Recovery and brain reorganization after stroke in adult and aged rats. Ann. Neurol. 58, 950–953 (2005).
Seymour, A. B. et al. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J. Cereb. Blood Flow Metab. 25, 1366–1375 (2005).
Oudega, M. & Hagg, T. Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord. Exp. Neurol. 140, 218–229 (1996).
Oudega, M. & Hagg, T. Neurotrophins promote regeneration of sensory axons in the adult rat spinal cord. Brain Res. 818, 431–438 (1999).
Neumann, S. & Woolf, C. J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23, 83–91 (1999).
Leon, S., Yin, Y., Nguyen, J., Irwin, N. & Benowitz, L. I. Lens injury stimulates axon regeneration in the mature rat optic nerve. J. Neurosci. 20, 4615–4626 (2000).
Steinmetz, M. P. et al. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J. Neurosci. 25, 8066–8076 (2005).
Wiesel, T. N. & Hubel, D. H. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017 (1963).
Pizzorusso, T. et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251 (2002). The first report describing the involvement of CSPGs in forming a perineuronal net that limits experience-driven plasticity in the adult.
McGee, A. W., Yang, Y., Fischer, Q. S., Daw, N. W. & Strittmatter, S. M. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 309, 2222–2226 (2005). Another important paper that further demonstrates the parallels between CNS myelin and CSPGs. It shows that myelin-associated inhibitors, such as CSPGs, are also involved in critical period closure.
Tropea, D., Caleo, M. & Maffei, L. Synergistic effects of brain-derived neurotrophic factor and chondroitinase ABC on retinal fiber sprouting after denervation of the superior colliculus in adult rats. J. Neurosci. 23, 7034–7044 (2003).
Massey, J. M. et al. Chondroitinase ABC digestion of the perineur onal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J. Neurosci. 26, 4406–4414 (2006). A study demonstrating that removal of CSPG inhibition also promotes functional collateral sprouting after injury, providing a potential mechanistic link between structural remodelling and functional plasticity.
Bareyre, F. M., Kerschensteiner, M., Misgeld, T. & Sanes, J. R. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nature Med. 11, 1355–1360 (2005).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Kerschensteiner, M., Schwab, M. E., Lichtman, J. W. & Misgeld, T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nature Med. 11, 572–577 (2005).
Lichtman, J. W. & Sanes, J. R. Watching the neuromuscular junction. J. Neurocytol. 32, 767–775 (2003).
Muller, C. M. & Best, J. Ocular dominance plasticity in adult cat visual cortex after transplantation of cultured astrocytes. Nature 342, 427–430 (1989).
Ferretti, P., Zhang, F. & O'Neill, P. Changes in spinal cord regenerative ability through phylogenesis and development: lessons to be learnt. Dev. Dyn. 226, 245–256 (2003).
Acknowledgements
We wish to thank M. Hou for critical reading of this manuscript. Our work is supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS), the McKnight foundation and the US National Multiple Sclerosis Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Dystrophic growth cones
-
Unusually shaped nerve terminals that are characterized by small globular clusters or multivesicular sacs found on the distal ends of regenerating axons in a glial scar environment.
- Dorsal root ganglia
-
(DRG). Ganglia that are found beside the spinal cord in which the cell bodies of sensory neurons are located. The bipolar neurons send a central axon through the spinal cord and another process to the PNS.
- Oligodendrocytes
-
Glial cells that elaborate myelin in the CNS. Unlike Schwann cells in the PNS that myelinate single axons, oligodendrocytes typically ensheath several processes at once.
- Astrocytes
-
The most abundant glial cell in the CNS, with a star-shaped cell body and broad end-feet on their processes. Astrocytes are thought to have nutritive functions, as well as roles in maintaining the blood–brain barrier and extracellular milieu.
- Glial scar
-
A physical and molecular barrier to regeneration that develops at CNS lesion sites, consisting primarily of reactive astrocytes, along with extracellular matrix molecules such as CSPGs.
- Growth cone
-
A motile actin-supported extension of a developing axon that can respond to external cues to guide its movement. Exposure to some repulsive guidance cues and many myelin-associated inhibitors leads to the collapse of this broad-shaped structure.
- Alternative splicing
-
A post-transcriptional process through which a pre-mRNA molecule, containing several introns and exons, can lead to different functional mRNA molecules, and consequently proteins, that originate from a single gene.
- GPI-anchor
-
A glycosylphosphatidylinositol (GPI) linkage, located at the carboxy termini of proteins without hydrophobic transmembrane regions, that can insert into the cell membrane. They might be released from the membrane by treatment with phospholipase C.
- Corticospinal tract
-
(CST). Axon fibres that originate from pyramidal neurons in layer 5 of the cerebral cortex and synapse on motor and interneurons in the spinal cord. This tract mediates motor functions and is commonly used for CNS injury models.
- Dorsal root entry zone
-
(DREZ). The interface between the CNS and PNS where sensory afferents from dorsal root ganglia enter the spinal cord during development.
- Penumbra
-
The area of secondary injury surrounding a CNS lesion epicentre.
- Dorsal rhizotomy
-
A transection of sensory nerve fibres in the dorsal root at its point of entry into the spinal cord.
- Rho-guanine dissociation inhibitor
-
(Rho-GDI). An inhibitory regulator of the Rho small G-protein family that can bind to RhoA and maintain it in an inactive state.
- Dorsal columns
-
Axon fibres that consist of the central processes of medium-diameter sensory dorsal root ganglia neurons that project up to dorsal column nuclei in the medulla.
- Raphespinal tract
-
Serotonin-containing fibres originating from caudal raphe nuclei in the brainstem to modulate sensory inputs such as pain.
- Rubrospinal tract
-
Axon fibres that are functionally related to corticospinal tracts, that originate from the caudal red nucleus and terminate on motor neurons in the spinal cord.
- Preconditioning injury
-
A lesion of the peripheral branch of bipolar sensory neurons in dorsal root ganglia that can promote the subsequent regeneration of their central axons in the spinal cord after nerve transection at a later time point.
Rights and permissions
About this article
Cite this article
Yiu, G., He, Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7, 617–627 (2006). https://doi.org/10.1038/nrn1956
Issue Date:
DOI: https://doi.org/10.1038/nrn1956
This article is cited by
-
Extracellular vesicles from UTX-knockout endothelial cells boost neural stem cell differentiation in spinal cord injury
Cell Communication and Signaling (2024)
-
Biomaterial-based regenerative therapeutic strategies for spinal cord injury
NPG Asia Materials (2024)
-
Anatomical measurements of trigeminal ganglion: a cadaver study
Anatomical Science International (2024)
-
Corticospinal tract: a new hope for the treatment of post-stroke spasticity
Acta Neurologica Belgica (2024)
-
Enhancing structural plasticity of PC12 neurons during differentiation and neurite regeneration with a catalytically inactive mutant version of the zRICH protein
BMC Neuroscience (2023)