Key Points
-
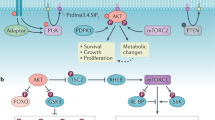
Metabolic pathways such as glycolysis, the tricarboxylic acid cycle and fatty acid synthesis influence key cell cycle and apoptotic effectors to promote cell survival or death and dictate cell fate.
-
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoenzyme 3 (PFKFB3) produces fructose-2,6-bisphosphate and promotes the expression of cyclin D3 and Cdc25C while inhibiting the expression of the cyclin-dependent kinase 1 (CDK1) inhibitor p27. In a reciprocal manner, cyclin D1 overexpression results in decreased expression of hexokinase II, pyruvate kinase, fatty acid synthase and acetyl-CoA carboxylase, thus influencing metabolism.
-
The anaphase-promoting complex–Cdc20 homologue 1 (APC–CDH1) is active from late mitosis and throughout G1 phase and has been shown to ubiquitylate PFKFB3, targeting it for degradation. APC–CDH1 activity results in decreased PFKFB3 levels, decreased glycolysis and a concomitant increase in glucose flux through the pentose phosphate pathway (PPP) to protect cells from oxidative stress-induced apoptosis.
-
ATP-citrate lyase (ACL) links glucose metabolism to replication and transcription by regulating citrate-derived acetyl-CoA. Glucose metabolism results in increased citrate production, which is used by ACL to generate acetyl-CoA, a substrate important for histone acetylation and the transcriptional upregulation of genes, including those involved in metabolism such as phosphofructokinase 1, hexokinase II, L-lactate dehydrogenase A chain and glucose transporter 4.
-
When intracellular glucose levels are low, p53 is phosphorylated by 5′-AMP-activated protein kinase (AMPK) to promote G1–S cell cycle arrest. p53 activity also influences apoptosis by regulating the expression of PUMA, NOXA, BAX and PIDD, and metabolism by regulating the expression of guanidinoacetate N-methyltransferase (GAMT), synthesis of cytochrome c oxidase 2 (SCO2), glutaminase 2, TP53-induced glycolysis and apoptosis regulator (TIGAR) and phosphoglycerate mutase (PGM).
-
In Xenopus laevis, caspase 2 is inhibited in response to glucose flux through the PPP and NADPH production, through phosphorylation mediated by metabolism-regulated Ca2+/calmodulin-dependent protein kinase II. Caspase 2 activation is also under metabolic control, as waning NADPH levels signal for 14-3-3 release from caspase 2, facilitating caspase 2 dephosphorylation by protein phosphatase 1. B cell lymphoma 2 (BCL-2) family proteins are extensively regulated by metabolism to create a threshold for mitochondrial outer membrane permeabilization and apoptosis. For example, pro-apoptotic BCL-2 antagonist of cell death (BAD) is phosphorylated and inhibited by AKT in response to increased glucose uptake; this prevents BAD-mediated apoptosis in the setting of sufficient nutrients and raises the threshold for apoptotic stimuli.
Abstract
Metabolic activity is a crucial determinant of a cell's decision to proliferate or die. Although it is not fully understood how metabolic pathways such as glycolysis and the pentose phosphate pathway communicate to cell cycle and apoptotic effectors, it is clear that a complex network of signalling molecules is required to integrate metabolic inputs. D-type cyclins, cyclin-dependent kinases, the anaphase-promoting complex, p53, caspase 2 and B cell lymphoma 2 proteins, among others, have been shown to be regulated by metabolic crosstalk. Elucidating these pathways is of great importance, as metabolic aberrations and their downstream effects are known to contribute to the aetiology of cancer and degenerative disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Salati, L. M. & Amir-Ahmady, B. Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu. Rev. Nutr. 21, 121–140 (2001).
Engelman, J. A., Luo, J. & Cantley, L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature Rev. Genet. 7, 606–619 (2006).
Manning, B. D. & Cantley, L. C. AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 (2007).
Hardie, D. G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature Rev. Mol. Cell Biol. 8, 774–785 (2007).
Wullschleger, S., Loewith, R. & Hall, M. N. TOR signaling in growth and metabolism. Cell 124, 471–484 (2006).
Nurse, P. A long twentieth century of the cell cycle and beyond. Cell 100, 71–78 (2000).
Sherr, C. J. G1 phase progression: cycling on cue. Cell 79, 551–555 (1994).
Coller, H. A., Sang, L. & Roberts, J. M. A new description of cellular quiescence. PLoS Biol. 4, e83 (2006).
Zwerschke, W. et al. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem. J. 376, 403–411 (2003).
Sherr, C. J. D-type cyclins. Trends Biochem. Sci. 20, 187–190 (1995).
Yalcin, A. et al. Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases. J. Biol. Chem. 284, 24223–24232 (2009). This work shows that unlike other PFKFB isoforms, PFKFB3 is localized to nuclei and that this nuclear localization is required for its ability to control cell cycle progression by altering the expression of multiple cell cycle regulators.
Sakamaki, T. et al. Cyclin D1 determines mitochondrial function in vivo. Mol. Cell. Biol. 26, 5449–5469 (2006). Shows that cyclin D1 also suppresses mitochondrial function and aerobic glycolysis by decreasing the expression of many metabolic enzymes.
Bienvenu, F. et al. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature 463, 374–378.
Glauser, D. A. & Schlegel, W. The FoxO/Bcl-6/cyclin D2 pathway mediates metabolic and growth factor stimulation of proliferation in Min6 pancreatic β-cells. J. Recept Signal Transduct Res. 29, 293–298 (2009).
Chesney, J. et al. An inducible gene product for 6-phosphofructo-2-kinase with an AU-rich instability element: role in tumor cell glycolysis and the Warburg effect. Proc. Natl Acad. Sci. USA 96, 3047–3052 (1999).
Okar, D. A. & Lange, A. J. Fructose-2, 6-bisphosphate and control of carbohydrate metabolism in eukaryotes. Biofactors 10, 1–14 (1999).
Yang, K., Hitomi, M. & Stacey, D. W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 1, 32 (2006).
Mandal, S., Freije, W. A., Guptan, P. & Banerjee, U. Metabolic control of G1-S transition: cyclin E degradation by p53-induced activation of the ubiquitin-proteasome system. J. Cell Biol. 188, 473–479 (2010).
Mandal, S., Guptan, P., Owusu-Ansah, E. & Banerjee, U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev. Cell 9, 843–854 (2005).
Li, M. & Zhang, P. The function of APC/CCdh1 in cell cycle and beyond. Cell Div. 4, 2 (2009).
Almeida, A., Bolanos, J. P. & Moncada, S. E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation. Proc. Natl Acad. Sci. USA 107, 738–741. Reports that PFKFB3 is a substrate of the APC and that glycolysis and cell proliferation are therefore dependent on a drop in APC activity.
Herrero-Mendez, A. et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nature Cell Biol. 11, 747–752 (2009).
Li, B., Carey, M. & Workman, J. L. The role of chromatin during transcription. Cell 128, 707–719 (2007).
Knosp, O., Talasz, H. & Puschendorf, B. Histone acetylation and histone synthesis in mouse fibroblasts during quiescence and restimulation into S-phase. Mol. Cell Biochem. 101, 51–58 (1991).
Wellen, K. E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009). Shows that acetyl-CoA generated by ACL is required for histone acetylation; control of ACL activity by glucose uptake therefore allows metabolic input into the histone acetylation required for DNA replication and transcription following growth factor stimulation.
Kaplan, R. S., Mayor, J. A. & Wood, D. O. The mitochondrial tricarboxylate transport protein. cDNA cloning, primary structure, and comparison with other mitochondrial transport proteins. J. Biol. Chem. 268, 13682–13690 (1993).
Annicotte, J. S. et al. The CDK4-pRB-E2F1 pathway controls insulin secretion. Nature Cell Biol. 11, 1017–1023 (2009).
Fajas, L. et al. Impaired pancreatic growth, β cell mass, and beta cell function in E2F1−/− mice. J. Clin. Invest. 113, 1288–95 (2004).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–219 (2004).
Nicholson, D. W. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6, 1028–1042 (1999).
Taylor, R. C., Cullen, S. P. & Martin, S. J. Apoptosis: controlled demolition at the cellular level. Nature Rev. Mol. Cell Biol. 9, 231–241 (2008).
Acehan, D. et al. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell 9, 423–432 (2002).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Schafer, Z. T. & Kornbluth, S. The apoptosome: physiological, developmental, and pathological modes of regulation. Dev. Cell 10, 549–561 (2006).
Zou, H., Henzel, W. J., Liu, X., Lutschg, A. & Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90, 405–413 (1997).
Jones, R. G. et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293 (2005).
Oda, E. et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 (2000).
Miyashita, T. & Reed, J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293–299 (1995).
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 (2001).
Chipuk, J. E., Bouchier-Hayes, L., Kuwana, T., Newmeyer, D. D. & Green, D. R. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science 309, 1732–1735 (2005).
Tinel, A. & Tschopp, J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304, 843–846 (2004).
Zhao, Y. et al. Glucose metabolism attenuates p53 and Puma-dependent cell death upon growth factor deprivation. J. Biol. Chem. 283, 36344–36353 (2008). Shows that cell death induced by PUMA is linked to nutrient levels, as high glucose metabolism can suppress p53-mediated PUMA induction even under conditions of growth factor withdrawal.
Mihara, M. et al. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11, 577–590 (2003).
Ide, T. et al. GAMT, a p53-inducible modulator of apoptosis, is critical for the adaptive response to nutrient stress. Mol. Cell 36, 379–392 (2009).
Kondoh, H. et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 65, 177–185 (2005).
Matoba, S. et al. p53 regulates mitochondrial respiration. Science 312, 1650–1653 (2006).
Bensaad, K. et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126, 107–120 (2006). Identifies a new p53-inducible gene, TIGAR, that can decrease intracellular levels of Fru-2,6-BP, thereby dampening glycolysis and protecting cells from reactive oxygen species-induced apoptosis through PPP-mediated generation of NADPH.
Hu, W. et al. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc. Natl Acad. Sci. USA 107, 7455–7460 (2010).
Suzuki, S. et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl Acad. Sci. USA 107, 7461–7466 (2010).
Baliga, B. C., Read, S. H. & Kumar, S. The biochemical mechanism of caspase-2 activation. Cell Death Differ. 11, 1234–1241 (2004).
Krumschnabel, G., Manzl, C. & Villunger, A. Caspase-2: killer, savior and safeguard — emerging versatile roles for an ill-defined caspase. Oncogene 28, 3093–3096 (2009).
Bergeron, L. et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 12, 1304–1314 (1998).
Nutt, L. K. et al. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell 123, 89–103 (2005). Shows that nutrients sufficient to promote the generation of NADPH through the PPP can promote NADPH-dependent suppressive phosphorylation of caspase 2 by CaMKII.
Nutt, L. K. et al. Metabolic control of oocyte apoptosis mediated by 14-3-3ζ-regulated dephosphorylation of caspase-2. Dev. Cell 16, 856–866 (2009).
Newmeyer, D. D., Farschon, D. M. & Reed, J. C. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell 79, 353–364 (1994).
Bouchier-Hayes, L. et al. Characterization of cytoplasmic caspase-2 activation by induced proximity. Mol. Cell 35, 830–840 (2009). This work employs bimolecular fluorescence complementation to demonstrate cytoplasmic activation of caspase 2 in response to several stimuli, including DHEA, an inhibitor of the PPP.
Andersen, J. L. et al. Restraint of apoptosis during mitosis through interdomain phosphorylation of caspase-2. EMBO J. 28, 3216–3227 (2009).
Shulga, N., Wilson-Smith, R. & Pastorino, J. G. Hexokinase II detachment from the mitochondria potentiates cisplatin induced cytotoxicity through a caspase-2 dependent mechanism. Cell Cycle 8, 3355–3364 (2009).
Ryan, S. D. et al. Amyloid-β42 signals tau hyperphosphorylation and compromises neuronal viability by disrupting alkylacylglycerophosphocholine metabolism. Proc. Natl Acad. Sci. USA 106, 20936–20941 (2009).
Majewski, N. et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell 16, 819–830 (2004).
Klein, J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J. Neural Transm. 107, 1027–1063 (2000).
Troy, C. M. et al. Caspase-2 mediates neuronal cell death induced by β-amyloid. J. Neurosci. 20, 1386–1392 (2000).
Tait, S. W. & Green, D. R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Rev. Mol. Cell Biol. 11, 621–632 (2010).
Danial, N. N. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin. Cancer Res. 13, 7254–7263 (2007).
del Peso, L., Gonzalez-Garcia, M., Page, C., Herrera, R. & Nunez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278, 687–689 (1997).
Datta, S. R. et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241 (1997).
Rathmell, J. C., Vander Heiden, M. G., Harris, M. H., Frauwirth, K. A. & Thompson, C. B. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell 6, 683–692 (2000).
Harada, H. et al. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell 3, 413–422 (1999).
Datta, S. R. et al. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev. Cell 3, 631–643 (2002).
Danial, N. N. et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 424, 952–956 (2003). Shows that BAD is required for assembly of a glucokinase-containing protein complex necessary both for mitochondrial glucokinase activity and optimal glucose-dependent mitochondrial respiratory function.
Alves, N. L. et al. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity 24, 703–716 (2006).
Rathmell, J. C. et al. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell Biol. 23, 7315–7328 (2003).
Yamaguchi, H. & Wang, H. G. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene 20, 7779–7786 (2001).
Pastorino, J. G., Shulga, N. & Hoek, J. B. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 277, 7610–7618 (2002).
Majewski, N., Nogueira, V., Robey, R. B. & Hay, N. Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol. Cell. Biol. 24, 730–740 (2004).
Zhao, Y. et al. Glycogen synthase kinase 3α and 3β mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol. Cell. Biol. 27, 4328–4339 (2007).
Maurer, U., Charvet, C., Wagman, A. S., Dejardin, E. & Green, D. R. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell 21, 749–760 (2006).
Jacobson, M. D. et al. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature 361, 365–369 (1993).
Wang, J., Silva, J. P., Gustafsson, C. M., Rustin, P. & Larsson, N. G. Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc. Natl Acad. Sci. USA 98, 4038–4043 (2001).
Ow, Y. P., Green, D. R., Hao, Z. & Mak, T. W. Cytochrome c: functions beyond respiration. Nature Rev. Mol. Cell Biol. 9, 532–542 (2008).
Wikstrom, M. K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature 266, 271–273 (1977).
Liu, X., Kim, C. N., Yang, J., Jemmerson, R. & Wang, X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147–157 (1996).
Schubert, D. Glucose metabolism and Alzheimer's disease. Ageing Res. Rev. 4, 240–257 (2005).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Vaughn, A. E. & Deshmukh, M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nature Cell Biol. 10, 1477–1483 (2008).
Colell, A. et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 129, 983–997 (2007). Shows that GAPDH supports survival in cells with permeabilized mitochondria by increasing glycolysis and, in a new nuclear role, increasing autophagy.
Rathmell, J. C. & Kornbluth, S. Filling a GAP(DH) in caspase-independent cell death. Cell 129, 861–863 (2007).
Green, D. R. & Kroemer, G. The pathophysiology of mitochondrial cell death. Science 305, 626–629 (2004).
Chipuk, J. E. & Green, D. R. Do inducers of apoptosis trigger caspase-independent cell death? Nature Rev. Mol. Cell Biol. 6, 268–275 (2005).
Majeski, A. E. & Dice, J. F. Mechanisms of chaperone-mediated autophagy. Int. J. Biochem. Cell Biol. 36, 2435–2444 (2004).
Colell, A., Green, D. R. & Ricci, J. E. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 16, 1573–1581 (2009).
Lartigue, L. et al. Caspase-independent mitochondrial cell death results from loss of respiration, not cytotoxic protein release. Mol. Biol. Cell 20, 4871–4884 (2009).
Chalfant, C. E. et al. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J. Biol. Chem. 277, 12587–12595 (2002).
Seol, D. W. & Billiar, T. R. A caspase-9 variant missing the catalytic site is an endogenous inhibitor of apoptosis. J. Biol. Chem. 274, 2072–2076 (1999).
Kim, H. S. et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 17, 41–52 (2010).
Xu, P., Vernooy, S. Y., Guo, M. & Hay, B. A. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr. Biol. 13, 790–795 (2003).
Yi, C. H. et al. A genome-wide RNAi screen reveals multiple regulators of caspase activation. J. Cell Biol. 179, 619–626 (2007).
Ruvolo, P. P., Deng, X., Ito, T., Carr, B. K. & May, W. S. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J. Biol. Chem. 274, 20296–300 (1999).
Ganesan, V. et al. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis 15, 553–562 (2010).
Siskind, L. J. et al. The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. J. Biol. Chem. 285, 11818–11826 (2010).
Acknowledgements
We are grateful to J. Rathmell and M. Kurokawa for critical reading and feedback on the manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Pentose phosphate pathway
-
A metabolic pathway that generates NADPH and pentose sugars from glucose-6-phosphate. NADPH is important for the biosynthesis of many cell components and serves as a major cellular antioxidant.
- Anaphase-promoting complex
-
A large multisubunit E3 ubiquitin ligase with a RING-containing subunit (APC11) that ubiquitylates, among other proteins, several proteins that are crucial for the transition from M phase to G1.
- Initiator caspase
-
A caspase lying at the apex of apoptotic signalling cascades (for example, caspase 2, caspase 8 and caspase 9). These cleave and activate executioner caspases.
- Executioner caspase
-
A caspase (caspase 3, caspase 6 and caspase 7) that cleaves a range of cellular substrate proteins, resulting in apoptotic cell death. Also termed effector caspases.
- Senescence
-
A cellular state of prolonged G1 cell cycle arrest with characteristic metabolic, morphological and protein expression alterations.
- Bimolecular fluorescence complementation
-
A method for detecting protein–protein interactions using non-fluorescent protein halves fused to the proteins of interest. When the proteins of interest interact, the non-fluorescent halves associate to form a fluorescent complex.
- Autophagy
-
A process in which intracellular contents are destroyed by bulk enclosure of cytoplasmic material in membrane-enclosed vesicles that are then targeted for lysosomal degradation.
Rights and permissions
About this article
Cite this article
Buchakjian, M., Kornbluth, S. The engine driving the ship: metabolic steering of cell proliferation and death. Nat Rev Mol Cell Biol 11, 715–727 (2010). https://doi.org/10.1038/nrm2972
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2972
This article is cited by
-
Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications
Journal of Hematology & Oncology (2022)
-
Cellular metabolic adaptations in rheumatoid arthritis and their therapeutic implications
Nature Reviews Rheumatology (2022)
-
DNA-based artificial molecular signaling system that mimics basic elements of reception and response
Nature Communications (2020)
-
Role of pyruvate kinase M2-mediated metabolic reprogramming during podocyte differentiation
Cell Death & Disease (2020)
-
The enhancement of glycolysis regulates pancreatic cancer metastasis
Cellular and Molecular Life Sciences (2020)