Key Points
-
Wiskott–Aldrich syndrome (WAS) is a rare X-linked disease that is characterized by immune dysregulation and micro-thrombocytopaenia.
-
WAS protein (WASp) is a haematopoietic-restricted member of a family of proteins that transduce signals from the cell surface to the actin cytoskeleton.
-
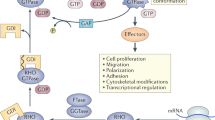
When activated by the Rho GTPase Cdc42, WASp binds to the cytoskeletal-organizing complex Arp2/3, which initiates polymerization of actin and formation of a branching network of filaments.
-
WASp has many effects on the immune system and participates in antigen-receptor signalling, phagocytosis and cell migration.
-
Many of the defects of WAS might be attributable to abnormal cell transport and cell–cell interactions.
Abstract
The regulation of many immunological events depends on systems that mediate dynamic actin reorganization in response to signals from the cell membrane. The Wiskott–Aldrich syndrome protein (WASp) is the founding member of a family of proteins that have emerged as crucial effectors of Rho GTPases and activators of the cytoskeletal-organizing complex Arp2/3. Now, WASp has been shown to be intimately involved in many pathways that influence the function of the immune system. Disturbances in these systems result in the complex immunodysregulation of Wiskott–Aldrich syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Symons, M. et al. Wiskott–Aldrich syndrome protein, a novel effector for the GTPase Cdc42Hs, is implicated in actin polymerization. Cell 84, 723–734 (1996).
Aspenstrom, P., Lindberg, U. & Hall, A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott–Aldrich syndrome. Curr. Biol. 6, 70–75 (1996).
Kolluri, R., Tolias, K. F., Carpenter, C. L., Rosen, F. S. & Kirchhausen, T. Direct interaction of the Wiskott–Aldrich syndrome protein with the GTPase Cdc42. Proc. Natl Acad. Sci. USA 93, 5615–5618 (1996).
Miki, H., Miura, K. & Takenawa, T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 15, 5326–5335 (1996).
Bear, J. E., Rawls, J. F. & Saxe, C. L. III. SCAR, a WASP-related protein, isolated as a suppressor of receptor defects in late Dictyostelium development. J. Cell Biol. 142, 1325–1335 (1998).
Miki, H., Suetsugu, S. & Takenawa, T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932–6941 (1998).
Suetsugu, S., Miki, H. & Takenawa, T. Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate with the Arp2/3 complex. Biochem. Biophys. Res. Commun. 260, 296–302 (1999).
Li, R. Bee1, a yeast protein with homology to Wiscott–Aldrich syndrome protein, is critical for the assembly of cortical actin cytoskeleton. J. Cell Biol. 136, 649–658 (1997).
Ben Yaacov, S., Le Borgne, R., Abramson, I., Schweisguth, F. & Schejter, E. D. Wasp, the Drosophila Wiskott–Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. J. Cell Biol. 152, 1–13 (2001).
Wiskott, A. Familiarer, angeborener Morbus Werlhofii? Monatsschr. Kinderheilkd. 68, 212–216 (1937).
Aldrich, R. A., Steinberg, A. G. & Campbell, D. C. Pedigree demonstrating a sex-linked recessive condition characterised by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics 13, 133–139 (1954).
Derry, J. M., Ochs, H. D. & Francke, U. Isolation of a novel gene mutated in Wiskott–Aldrich syndrome. Cell 79, 922 (1994).
Villa, A. et al. X-linked thrombocytopenia and Wiskott–Aldrich syndrome are allelic diseases with mutations in the WASP gene. Nature Genet. 9, 414–417 (1995).
Zhu, Q. et al. The Wiskott–Aldrich syndrome and X-linked congenital thrombocytopenia are caused by mutations of the same gene. Blood 86, 3797–3804 (1995).
Ochs, H., Smith, C. I. E. & Puck, J. M. Primary Immunodeficiency Diseases (Oxford University Press, New York, 1999).
Renfranz, P. J. & Beckerle, M. C. Doing (F/L)PPPPs: EVH1 domains and their proline-rich partners in cell polarity and migration. Curr. Opin. Cell Biol. 14, 88–103 (2002).
Rohatgi, R., Ho, H. Y. & Kirschner, M. W. Mechanism of N-WASP activation by Cdc42 and phosphatidylinositol-4,5-bisphosphate. J. Cell Biol. 150, 1299–1310 (2000).
Higgs, H. N. & Pollard, T. D. Activation by Cdc42 and PIP2 of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol. 150, 1311–1320 (2000).
Ramesh, N., Anton, I. M., Hartwig, J. H. & Geha, R. S. WIP, a protein associated with Wiskott–Aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl Acad. Sci. USA 94, 14671–14676 (1997).
Stewart, D. M., Tian, L. & Nelson, D. L. Mutations that cause the Wiskott–Aldrich syndrome impair the interaction of Wiskott–Aldrich syndrome protein (WASP) with WASP-interacting protein. J. Immunol. 162, 5019–5024 (1999).
Martinez-Quiles, N. et al. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nature Cell Biol. 3, 484–491 (2001).
Bar-Sagi, D. & Hall, A. Ras and Rho GTPases: a family reunion. Cell 103, 227–238 (2000).
Kozma, R., Ahmed, S., Best, A. & Lim, L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell Biol. 15, 1942–1952 (1995).
Nobes, C. D. & Hall, A. Rho, rac and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell 81, 53–62 (1995).
Allen, W. E., Jones, G. E., Pollard, J. W. & Ridley, A. J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J. Cell Sci. 110, 707–720 (1997).
Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D. & Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 (1992).
Lamarche, N. et al. Rac and Cdc42 induce actin polymerization and G1 cell-cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87, 519–529 (1996).
Rottner, K., Hall, A. & Small, J. V. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9, 640–648 (1999).
Corre, I., Gomez, M., Vielkind, S. & Cantrell, D. A. Analysis of thymocyte development reveals that the GTPase RhoA is a positive regulator of T-cell receptor responses in vivo. J. Exp. Med. 194, 903–914 (2001).
Ridley, A. J. Rho proteins: linking signaling with membrane trafficking. Traffic. 2, 303–310 (2001).
Wu, W. J., Erickson, J. W., Lin, R. & Cerione, R. A. The γ-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature 405, 800–804 (2000).
Aspenstrom, P. Effectors for the Rho GTPases. Curr. Opin. Cell Biol. 11, 95–102 (1999).
Bishop, A. L. & Hall, A. Rho GTPases and their effector proteins. Biochem. J. 348, 241–255 (2000).
Rudolph, M. G. et al. The Cdc42/Rac interactive binding-region motif of the Wiskott–Aldrich syndrome protein (WASP) is necessary but not sufficient for tight binding to Cdc42 and structure formation. J. Biol. Chem. 273, 18067–18076 (1998).
Miki, H., Yamaguchi, H., Suetsugu, S. & Takenawa, T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature 408, 732–735 (2000).
Prehoda, K. E., Scott, J. A., Mullins, R. D. & Lim, W. A. Integration of multiple signals through cooperative regulation of the N-WASP–Arp2/3 complex. Science 290, 801–806 (2000).
Kim, A. S., Kakalis, L. T., Abdul-Manan, N., Liu, G. A. & Rosen, M. K. Autoinhibition and activation mechanisms of the Wiskott–Aldrich syndrome protein. Nature 404, 151–158 (2000).Contact between the GTPase-binding domain and the carboxyl terminus of WASp results in occlusion of the Arp2/3-binding site. This mechanism is presumed to regulate WASp activity.
Rohatgi, R. et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 (1999).
Carlier, M. F. et al. GRB2 links signalling to actin assembly by enhancing interaction of neural Wiskott–Aldrich syndrome protein (N-WASP) with actin-related-protein (ARP2/3) complex. J. Biol. Chem. 275, 21946–21952 (2000).
Rohatgi, R., Nollau, P., Ho, H. Y., Kirschner, M. W. & Mayer, B. J. Nck and phosphatidylinositol-4,5-bisphosphate synergistically activate actin polymerization through the N-WASP–Arp2/3 pathway. J. Biol. Chem. 276, 26448–26452 (2001).
Egile, C. et al. Activation of the Cdc42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146, 1319–1332 (1999).
Czech, M. P. PIP2 and PIP3: complex roles at the cell surface. Cell 100, 603–606 (2000).
Machesky, L. M. & Insall, R. H. Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8, 1347–1356 (1998).The Arp2/3 complex is regulated by WASp-family proteins through a conserved sequence domain.
Welch, M. D., DePace, A. H., Verma, S., Iwamatsu, A. & Mitchison, T. J. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J. Cell Biol. 138, 375–384 (1997).
Machesky, L. M. & Gould, K. L. The Arp2/3 complex: a multifunctional actin organizer. Curr. Opin. Cell Biol. 11, 117–121 (1999).
Mullins, R. D. & Pollard, T. D. Structure and function of the Arp2/3 complex. Curr. Opin. Struct. Biol. 9, 244–249 (1999).
Amann, K. J. & Pollard, T. D. The Arp2/3 complex nucleates actin filament branches from the sides of pre-existing filaments. Nature Cell Biol. 3, 306–310 (2001).The Arp2/3 complex is shown to preferentially nucleate actin-filament branches on the side of pre-existing filaments, rather than from the free barbed end of a filament.
Volkmann, N. et al. Structure of Arp2/3 complex in its activated state and in actin filament branch junctions. Science 293, 2456–2459 (2001).
Mullins, R. D., Heuser, J. A. & Pollard, T. D. The interaction of Arp2/3 complex with actin: nucleation, high-affinity pointed end capping and formation of branching networks of filaments. Proc. Natl Acad. Sci. USA 95, 6181–6186 (1998).
Blanchoin, L. et al. Direct observation of dendritic actin-filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404, 1007–1011 (2000).
Welch, M. D., Iwamatsu, A. & Mitchison, T. J. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature 385, 265–269 (1997).
Welch, M. D., Rosenblatt, J., Skoble, J., Portnoy, D. A. & Mitchison, T. J. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science 281, 105–108 (1998).
Loisel, T. P., Boujemaa, R., Pantaloni, D. & Carlier, M. F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401, 613–616 (1999).
Snapper, S. B. et al. N-WASP deficiency reveals distinct pathways for cell-surface projections and microbial actin-based motility. Nature Cell Biol. 3, 897–904 (2001).
Suzuki, T., Miki, H., Takenawa, T. & Sasakawa, C. Neural Wiskott–Aldrich syndrome protein is implicated in the actin-based motility of Shigella flexneri. EMBO J. 17, 2767–2776 (1998).
Moreau, V. et al. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nature Cell Biol. 2, 441–448 (2000).
Kalman, D. et al. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nature Cell Biol. 1, 389–391 (1999).
Gruenheid, S. et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nature Cell Biol. 3, 856–859 (2001).
Cudmore, S., Cossart, P., Griffiths, G. & Way, M. Actin-based motility of vaccinia virus. Nature 378, 636–638 (1995).
Rietdorf, J. et al. Kinesin-dependent movement on microtubules precedes actin-based motility of vaccinia virus. Nature Cell Biol. 3, 992–1000 (2001).
Lommel, S. et al. Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. 2, 850–857 (2001).
Krugmann, S. et al. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr. Biol. 11, 1645–1655 (2001).
Miki, H., Sasaki, T., Takai, Y. & Takenawa, T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 391, 93–96 (1998).
Lei, M. et al. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 102, 387–397 (2000).
Yang, C. et al. Profilin enhances Cdc42-induced nucleation of actin polymerization. J. Cell Biol. 150, 1001–1012 (2000).
Castellano, F., Le Clainche, C., Patin, D., Carlier, M. F. & Chavrier, P. A WASp–VASP complex regulates actin polymerization at the plasma membrane. EMBO J. 20, 5603–5614 (2001).
Baba, Y. et al. Involvement of Wiskott–Aldrich syndrome protein in B-cell cytoplasmic tyrosine kinase pathway. Blood 93, 2003–2012 (1999).
Cote, J. F. et al. PSTPIP is a substrate of PTP-PEST and serves as a scaffold guiding PTP-PEST toward a specific dephosphorylation of WASP. J. Biol. Chem. 277, 2973–2986 (2002).
Guinamard, R., Aspenstrom, P., Fougereau, M., Chavrier, P. & Guillemot, J. C. Tyrosine phosphorylation of the Wiskott–Aldrich syndrome protein by Lyn and Btk is regulated by Cdc42. FEBS Lett. 434, 431–436 (1998).
Banin, S., Gout, I. & Brickell, P. Interaction between Wiskott–Aldrich syndrome protein (WASP) and the Fyn protein-tyrosine kinase. Mol. Biol. Rep. 26, 173–177 (1999).
Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
Dustin, M. L. & Chan, A. C. Signaling takes shape in the immune system. Cell 103, 283–294 (2000).
Acuto, O. & Cantrell, D. T-cell activation and the cytoskeleton. Annu. Rev. Immunol. 18, 165–184 (2000).
Shaw, A. S. FERMing up the synapse. Immunity 15, 683–686 (2001).
Villalba, M. et al. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid-raft clustering in T cells. J. Cell Biol. 155, 331–338 (2001).
Stinchcombe, J. C., Bossi, G., Booth, S. & Griffiths, G. M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001).
Lee, K. H. et al. T-cell receptor signaling precedes immunological synapse formation. Science 295, 1539–1542 (2002).
Kenney, D. et al. Morphological abnormalities in the lymphocytes of patients with the Wiskott–Aldrich syndrome. Blood 68, 1329–1332 (1986).
Simon, H. U., Mills, G. B., Hashimoto, S. & Siminovitch, K. A. Evidence for defective transmembrane signaling in B cells from patients with Wiskott–Aldrich syndrome. J. Clin. Invest. 90, 1396–1405 (1992).
Molina, I. J., Sancho, J., Terhorst, C., Rosen, F. S. & Remold-O'Donnell, E. T cells of patients with the Wiskott–Aldrich syndrome have a restricted defect in proliferative responses. J. Immunol. 151, 4383–4390 (1993).
Gallego, M. D., Santamaria, M., Pena, J. & Molina, I. J. Defective actin reorganization and polymerization of Wiskott–Aldrich T cells in response to CD3-mediated stimulation. Blood 90, 3089–3097 (1997).
Cannon, J. L. et al. Wasp recruitment to the T-cell:APC contact site occurs independently of Cdc42 activation. Immunity 15, 249–259 (2001).Localization of WASp to the immunological synapse is independent of the GTPase-binding domain, but requires the proline-rich domain.
Snapper, S. B. et al. Wiskott–Aldrich syndrome protein-deficient mice reveal a role for WASP in T- but not B-cell activation. Immunity 9, 81–91 (1998).WASp-deficient mice that are generated by gene targeting do not develop features of classical WAS. However, they have decreased numbers of peripheral lymphocytes, and their T cells show impaired proliferation and cap formation when stimulated with anti-CD3 antibody.
Zhang, J. et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott–Aldrich syndrome protein-deficient lymphocytes. J. Exp. Med. 190, 1329–1342 (1999).WASp-deficient mice have defects in thymocyte development and cytoskeletal rearrangement after antigen-receptor activation.
Zhang, J. et al. WASp verprolin homology, cofilin homology, and acidic region domain–mediated actin polymerization is required for T-cell development. Proc. Natl Acad. Sci. USA 99, 2240–2245 (2002).Transgenic studies indicate that WASp is essential for normal thymocyte development and that actin polymerization is crucial for this activity.
Sato, M. et al. Overexpression of the Wiskott–Aldrich syndrome protein N-terminal domain in transgenic mice inhibits T-cell proliferative responses via TCR signaling without affecting cytoskeletal rearrangements. J. Immunol. 167, 4701–4709 (2001).
Krause, M. et al. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T-cell receptor (TCR) signaling to the actin cytoskeleton. J. Cell Biol. 149, 181–194 (2000).
Fischer, K. D. et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 8, 554–562 (1998).
Holsinger, L. J. et al. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 8, 563–572 (1998).
Villalba, M. et al. A novel functional interaction between Vav and PKCτ is required for TCR-induced T-cell activation. Immunity. 12, 151–160 (2000).
Krawczyk, C. et al. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity 16, 331–343 (2002).Vav1 and WASp are implicated in the formation of the immunological synapse. Polarized clustering of TCR, but not of integrins, seems to be dependent on WASp.
McGavin, M. K. et al. The intersectin-2 adaptor links Wiskott–Aldrich syndrome protein (WASp)-mediated actin polymerization to T-cell antigen receptor endocytosis. J. Exp. Med. 194, 1777–1787 (2001).
Facchetti, F. et al. Defective actin polymerization in EBV-transformed B-cell lines from patients with the Wiskott–Aldrich syndrome. J. Pathol. 185, 99–107 (1998).
Westerberg, L., Greicius, G., Snapper, S. B., Aspenstrom, P. & Severinson, E. Cdc42, Rac1 and the Wiskott–Aldrich syndrome protein are involved in the cytoskeletal regulation of B lymphocytes. Blood 98, 1086–1094 (2001).
Guinamard, R., Okigaki, M., Schlessinger, J. & Ravetch, J. V. Absence of marginal-zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nature Immunol. 1, 31–36 (2000).
Martin, F., Oliver, A. M. & Kearney, J. F. Marginal-zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14, 617–629 (2001).
Vermi, W. et al. The spleen in the Wiskott–Aldrich syndrome: histopathologic abnormalities of the white pulp correlate with the clinical phenotype of the disease. Am. J. Surg. Pathol. 23, 182–191 (1999).
Anton, I. M. et al. WIP deficiency reveals a differential role for WIP and the actin cytoskeleton in T- and B-cell activation. Immunity 16, 193–204 (2002).WIP is required for normal T-cell activation and formation of the immunological synapse.
Allen, W. E., Zicha, D., Ridley, A. J. & Jones, G. E. A role for Cdc42 in macrophage chemotaxis. J. Cell Biol. 141, 1147–1157 (1998).
Zicha, D. et al. Chemotaxis of macrophages is abolished in the Wiskott–Aldrich syndrome. Br. J. Haematol. 101, 659–665 (1998).
Badolato, R. et al. Monocytes from Wiskott–Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J. Immunol. 161, 1026–1033 (1998).
Binks, M. et al. Intrinsic dendritic-cell abnormalities in Wiskott–Aldrich syndrome. Eur. J. Immunol. 28, 3259–3267 (1998).Dendritic cells from WAS patients are devoid of podosomes and have marked defects of motility in vitro , which indicates that dysregulated cell transport in vivo contributes to the complex immunophenotype of WAS.
Burns, S., Thrasher, A. J., Blundell, M. P., Machesky, L. & Jones, G. E. Configuration of human dendritic-cell cytoskeleton by Rho GTPases, Wiskott–Aldrich syndrome protein and differentiation. Blood 98, 1142–1149 (2001).
Linder, S. et al. The polarization defect of Wiskott–Aldrich syndrome macrophages is linked to dislocalization of the Arp2/3 complex. J. Immunol. 165, 221–225 (2000).
Gavazzi, I., Nermut, M. V. & Marchisio, P. C. Ultrastructure and gold-immunolabelling of cell-substratum adhesions (podosomes) in RSV-transformed BHK cells. J. Cell Sci. 94, 85–99 (1989).
Defife, K. M., Jenney, C. R., Colton, E. & Anderson, J. M. Cytoskeletal and adhesive structural polarizations accompany IL-13-induced human macrophage fusion. J. Histochem. Cytochem. 47, 65–74 (1999).
Correia, I., Chu, D., Chou, Y. H., Goldman, R. D. & Matsudaira, P. Integrating the actin and vimentin cytoskeletons. Adhesion-dependent formation of fimbrin–vimentin complexes in macrophages. J. Cell Biol. 146, 831–842 (1999).
Marchisio, P. C. et al. Cell–substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J. Cell Biol. 99, 1696–1705 (1984).
Turksen, K., Kanehisa, J., Opas, M., Heersche, J. N. & Aubin, J. E. Adhesion patterns and cytoskeleton of rabbit osteoclasts on bone slices and glass. J. Bone Miner. Res. 3, 389–400 (1988).
Zambonin-Zallone, A. et al. Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: a β3 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp. Cell Res. 182, 645–652 (1989).
Lakkakorpi, P. T. & Vaananen, H. K. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J. Bone Miner. Res. 6, 817–826 (1991).
Teti, A., Marchisio, P. C. & Zallone, A. Z. Clear zone in osteoclast function: role of podosomes in regulation of bone-resorbing activity. Am. J. Physiol. 261, C1–C7 (1991).
Haddad, E. et al. The interaction between Cdc42 and WASP is required for SDF-1-induced T-lymphocyte chemotaxis. Blood 97, 33–38 (2001).
Wengler, G., Gorlin, J. B., Williamson, J. M., Rosen, F. S. & Bing, D. H. Nonrandom inactivation of the X chromosome in early-lineage hematopoietic cells in carriers of Wiskott–Aldrich syndrome. Blood 85, 2471–2477 (1995).
Marshall, C. J. & Thrasher, A. J. The embryonic origins of human haematopoiesis. Brit. J. Haematol. 112, 838–850 (2001).
Haddad, E. et al. The thrombocytopenia of Wiskott–Aldrich syndrome is not related to a defect in proplatelet formation. Blood 94, 509–518 (1999).
Chimini, G. & Chavrier, P. Function of Rho-family proteins in actin dynamics during phagocytosis and engulfment. Nature Cell Biol. 2, E191–E196 (2000).
Massol, P., Montcourrier, P., Guillemot, J. C. & Chavrier, P. Fc receptor-mediated phagocytosis requires Cdc42 and Rac1. EMBO J. 17, 6219–6229 (1998).
Caron, E. & Hall, A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717–1721 (1998).
Cox, D. et al. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 186, 1487–1494 (1997).
Lorenzi, R., Brickell, P. M., Katz, D. R., Kinnon, C. & Thrasher, A. J. Wiskott–Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood 95, 2943–2946 (2000).
May, R. C., Caron, E., Hall, A. & Machesky, L. M. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nature Cell Biol. 2, 246–248 (2000).
Botto, M. et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature Genet. 19, 56–59 (1998).
Leverrier, Y. et al. Cutting edge: the Wiskott–Aldrich syndrome protein is required for efficient phagocytosis of apoptotic cells. J. Immunol. 166, 4831–4834 (2001).
Garrett, W. S. et al. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102, 325–334 (2000).
West, M. A., Prescott, A. R., Eskelinen, E. L., Ridley, A. J. & Watts, C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr. Biol. 10, 839–848 (2000).
Ariga, T. et al. Spontaneous in vivo reversion of an inherited mutation in the Wiskott–Aldrich syndrome. J. Immunol. 166, 5245–5249 (2001).
Wada, T. et al. Somatic mosaicism in Wiskott–Aldrich syndrome suggests in vivo reversion by a DNA slippage mechanism. Proc. Natl Acad. Sci. USA 98, 8697–8702 (2001).
Notarangelo, L. D. et al. Missense mutations of the WASP gene cause intermittent X-linked thrombocytopenia. Blood 99, 2268–2269 (2002).
Devriendt, K. et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nature Genet. 27, 313–317 (2001).Mutations in the GBD of WASp are shown to produce a clinical disorder that is distinct from WAS and characterized by neutropaenia. Disruption of the autoinhibitory domain results in enhanced and, therefore, dysregulated WASp activity.
Vignal, E. et al. Characterization of TCL, a new GTPase of the Rho family related to TC10 and Cdc42. J. Biol. Chem. 275, 36457–36464 (2000).
Neudauer, C. L., Joberty, G., Tatsis, N. & Macara, I. G. Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr. Biol. 8, 1151–1160 (1998).
Rivero-Lezcano, O. M., Marcilla, A., Sameshima, J. H. & Robbins, K. C. Wiskott–Aldrich syndrome protein physically associates with Nck through Src-homology 3 domains. Mol. Cell Biol. 15, 5725–5731 (1995).
She, H. Y. et al. Wiskott–Aldrich syndrome protein is associated with the adapter protein Grb2 and the epidermal growth factor receptor in living cells. Mol. Biol. Cell 8, 1709–1721 (1997).
Banin, S. et al. Wiskott–Aldrich syndrome protein (WASp) is a binding partner for c-Src family protein-tyrosine kinases. Curr. Biol. 6, 981–988 (1996).
Wu, Y., Spencer, S. D. & Lasky, L. A. Tyrosine phosphorylation regulates the SH3-mediated binding of the Wiskott–Aldrich syndrome protein to PSTPIP, a cytoskeletal-associated protein. J. Biol. Chem. 273, 5765–5770 (1998).
Suetsugu, S., Miki, H. & Takenawa, T. The essential role of profilin in the assembly of actin for microspike formation. EMBO J. 17, 6516–6526 (1998).
Tian, L., Nelson, D. L. & Stewart, D. M. Cdc42-interacting protein 4 mediates binding of the Wiskott–Aldrich syndrome protein to microtubules. J. Biol. Chem. 275, 7854–7861 (2000).
Marchand, J. B., Kaiser, D. A., Pollard, T. D. & Higgs, H. N. Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nature Cell Biol. 3, 76–82 (2001).
Chen, F. et al. Cdc42 is required for PIP2-induced actin polymerization and early development, but not for cell viability. Curr. Biol. 10, 758–765 (2000).
Acknowledgements
I would like to thank Professors G. Jones and C. Kinnon for many helpful discussions; S. De Noronha, S. Barker and S. Burns for confocal images; and S. Ballard for help with the preparation of this manuscript. I am also grateful to the Wellcome Trust, the Primary Immunodeficiency Association and the European Union 5th Framework for continued funding.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Entrez
FlyBase
InterPro
LocusLink
OMIM
<i>Saccharomyces</i> Genome Database
Swiss-Prot
Glossary
- THROMBOCYTOPAENIA
-
A reduced number of circulating platelets, owing to either the failure of production from bone-marrow megakaryocytes or increased clearance from the circulation, predominantly in the spleen. Thrombocytopaenia with small platelet volume is known as micro-thrombocytopaenia.
- IMMUNOLOGICAL SYNAPSE
-
A structure that is formed on the cell surface between a T cell and an antigen-presenting cell or a target cell; also known as a supra-molecular activation cluster. Important molecules that are involved in T-cell activation — including the T-cell receptor, many signal-transduction molecules and molecular adaptors — accumulate at this site. Mobilization of the actin cytoskeleton of the cell is required for synapse formation.
- CAPPING
-
The formation of an asymmetric patch of molecules — including adhesion molecules, and T-cell receptors or B-cell receptors — on the lymphocyte surface after stimulation.
- X-INACTIVATION
-
In females, a single, randomly selected X-chromosome is inactivated in each cell during early embryogenesis to avoid an imbalance of X-linked genes. This process is controlled by the Xist gene, which transcribes to a large non-protein-encoding RNA and triggers widespread gene silencing on the same X-chromosome. In some cases, mutations in Xist result in inactivation of only one of the X-chromosomes so that the pattern is non-random. If one of the X-chromosomes encodes a gene that has detrimental effects on cell growth or survival, development of cells with the normal chromosome will be favoured. This is known as apparent non-random X-inactivation.
Rights and permissions
About this article
Cite this article
Thrasher, A. Wasp in immune-system organization and function. Nat Rev Immunol 2, 635–646 (2002). https://doi.org/10.1038/nri884
Issue Date:
DOI: https://doi.org/10.1038/nri884
This article is cited by
-
Inadequate Activation of γδT- and B-cells in Patient with Wiskott-Aldrich Syndrome (WAS) Portrayed by TRG and IGH Repertoire Analyses
Journal of Clinical Immunology (2023)
-
The balance between the intronic miR-342 and its host gene Evl determines hematopoietic cell fate decision
Leukemia (2021)
-
First case of neutropenia and thrombocytopenia in the setting of cerebral cavernous malformation 3
International Journal of Hematology (2019)
-
Alternative polyadenylation factors link cell cycle to migration
Genome Biology (2018)
-
Hematopoietic stem cell gene therapy for the cure of blood diseases: primary immunodeficiencies
Rendiconti Lincei. Scienze Fisiche e Naturali (2018)