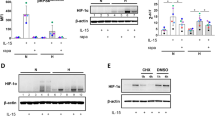
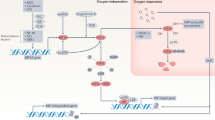
Key Points
-
It should be appreciated that the oxygen tensions in inflamed tissues, and even in normal tissues, are usually low (that is, these tissues are hypoxic).
-
The molecular mechanisms that ensure adaptation to hypoxia are operational in immune cells, thereby enabling immune surveillance in all tissue microenvironments and preventing 'safe shelters' for pathogens.
-
Activated immune cells mainly rely on glycolysis, rather than on oxidative phosphorylation, as a source of energy.
-
Hypoxia can regulate the activity of immune cells by promoting accumulation of adenosine and by stabilizing hypoxia-inducible factor 1α (HIF1α).
-
Inhibition of HIF1 might have different effects in various types of immune cell. HIF1α deficiency in myeloid cells results in reduced inflammation. By contrast, in T cells, HIF1α is thought to be a negative regulator of activity.
-
HIF1 is a promising therapeutic target for modulating immune responses. Inhibition or upregulation of HIF1α might induce or reduce inflammation depending on the type of immune response.
Abstract
Immune cells are often exposed to low oxygen tensions, which markedly affect cellular metabolism. We describe how activated T cells adapt to the changing energy supplies in hypoxic areas of inflamed tissues by using hypoxia-inducible factor 1 (HIF1) to switch to glycolysis as the main source of energy and by signalling through extracellular-adenosine receptors. This hypoxic regulation might alter the balance between T helper 1 cells and T helper 2 cells and might alter the activities of cells of the innate immune system, thereby qualitatively and quantitatively affecting immune responses. This regulatory mechanism should be taken into account in the design and interpretation of in vitro and in vivo studies of immune-cell effector functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sitkovsky, M. V. et al. Physiological control of immune response and inflammatory tissue damage by hypoxia inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 22, 657–682 (2004).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003). Using the Cre– loxP system with Cre expression under control of the lysozyme M promoter, the authors showed that HIF1α deficiency in macrophages causes metabolic defects as a consequence of inhibited glycolysis and results in an impaired inflammatory response.
Kojima, H. et al. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1α-deficient chimeric mice. Proc. Natl Acad. Sci. USA 99, 2170–2174 (2002). Using the RAG2-deficient blastocyst-complementation system, the authors examined HIF1α deficiency in T and B cells and showed that loss of HIF1α results in defects in the B-cell lineage, autoimmunity and tissue damage.
Caldwell, C. C. et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J. Immunol. 167, 6140–6149 (2001).
Lukashev, D., Caldwell, C., Ohta, A., Chen, P. & Sitkovsky, M. Differential regulation of two alternatively spliced isoforms of hypoxia-inducible factor-1α in activated T lymphocytes. J. Biol. Chem. 276, 48754–48763 (2001).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Roman, E. et al. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 196, 957–968 (2002).
Reinhardt, R. L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M. K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Baron, V. et al. The repertoires of circulating human CD8+ central and effector memory T cell subsets are largely distinct. Immunity 18, 193–204 (2003).
Wherry, E. J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature Immunol. 4, 225–234 (2003).
Wang, G. L. & Semensa, G. L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl Acad. Sci. USA 90, 4304–4308 (1993).
Linden, J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 41, 775–787 (2001).
Loeffler, D. A., Keng, P. C., Baggs, R. B. & Lord, E. M. Lymphocytic infiltration and cytotoxicity under hypoxic conditions in the EMT6 mouse mammary tumor. Int. J. Cancer 45, 462–467 (1990).
Dewhirst, M. W. Concepts of oxygen transport at the microcirculatory level. Semin. Radiat. Oncol. 8, 143–150 (1998).
Van Belle, H., Goossens, F. & Wynants, J. Formation and release of purine catabolites during hypoperfusion, anoxia, and ischemia. Am. J. Pathol. 252, H886–H893 (1987).
Matherne, G. P., Headrick, J. P., Coleman, S. D. & Berne, R. M. Interstitial transudate purines in normoxic and hypoxic immature and mature rabbit hearts. Pediatr. Res. 28, 348–353 (1990).
Helmlinger, G., Yuan, F., Dellian, M. & Jain, R. K. Interstitial pH and Po2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nature Med. 3, 177–182 (1997).
Vaupel, P. W., Frinak, S. & Bicher, H. I. Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Res. 41, 2008–2013 (1981).
Arnold, F., West, D. & Kumar, S. Wound healing: the effect of macrophage and tumour derived angiogenesis factors on skin graft vascularization. Br. J. Exp. Pathol. 68, 569–574 (1987).
Simmen, H. P., Battaglia, H., Giovanoli, P. & Blaser, J. Analysis of pH, Po2 and Pco2 in drainage fluid allows for rapid detection of infectious complications during the follow-up period after abdominal surgery. Infection 22, 386–389 (1994).
Thiel, M. et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 3, e174 (2005). This study established that both hypoxia and A 2A Rs are required for tissue protection and that interference in this pathway might exacerbate human disease.
Vanderkooi, J. M., Erecinska, M. & Silver, I. A. Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am. J. Physiol. 260, C1131–C1150 (1991).
Vaupel, P., Braunbeck, W. & Thews, G. Respiratory gas exchange and Po2-distribution in splenic tissue. Adv. Exp. Med. Biol. 37A, 401–406 (1973).
Braun, R. D., Lanzen, J. L., Snyder, S. A. & Dewhirst, M. W. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am. J. Physiol. Heart Circ. Physiol. 280, H2533–H2544 (2001).
Volkholz, H. J. et al. Measurement of local Po2 and intracapillary hemoglobin oxygenation in lung tissue of rabbits. Adv. Exp. Med. Biol. 169, 633–641 (1984).
Buttgereit, F., Burmester, G. R. & Brand, M. D. Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol. Today 21, 192–199 (2000).
Saraste, M. Oxidative phosphorylation at the fin de siècle. Science 283, 1488–1493 (1999).
Warburg, O. On respiratory impairment in cancer cells. Science 124, 269–270 (1956).
McKeehan, W. L. Glycolysis, glutaminolysis and cell proliferation. Cell Biol. Int. Rep. 6, 635–650 (1982).
Wang, T., Marquardt, C. & Foker, J. Aerobic glycolysis during lymphocyte proliferation. Nature 261, 702–705 (1976).
Levene, P. A. & Meyer, G. M. The action of leukocytes on glucose. J. Biol. Chem. 11, 361–370 (1912).
Borregaard, N. & Herlin, T. Energy metabolism of human neutrophils during phagocytosis. J. Clin. Invest. 70, 550–557 (1982).
Rathmell, J. C., Vander Heiden, M. G., Harris, M. H., Frauwirth, K. A. & Thompson, C. B. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell 6, 683–692 (2000).
Krauss, S., Brand, M. D. & Buttgereit, F. Signaling takes a breath — new quantitative perspectives on bioenergetics and signal transduction. Immunity 15, 497–502 (2001).
Roos, D. & Loos, J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Exp. Cell Res. 77, 127–135 (1973).
Hume, D. A., Radik, J. L., Ferber, E. & Weidemann, M. J. Aerobic glycolysis and lymphocyte transformation. Biochem. J. 174, 703–709 (1978).
Greiner, E. F., Guppy, M. & Brand, K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J. Biol. Chem. 269, 31484–31490 (1994).
Brand, K. A. & Hermfisse, U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 11, 388–395 (1997).
O'Flaherty, J. T., Kreutzer, D. L., Showell, H. J. & Ward, P. A. Influence of inhibitors of cellular function on chemotactic factor-induced neutrophil aggregation. J. Immunol. 119, 1751–1756 (1977).
Simchowitz, L., Mehta, J. & Spilberg, I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: effect of metabolic inhibitors and antiinflammatory drugs. Arthritis Rheum. 22, 755–763 (1979).
MacDonald, H. R. & Koch, C. J. Energy metabolism and T cell-mediated cytolysis. I. Synergism between inhibitors of respiration and glycolysis. J. Exp. Med. 146, 698–709 (1977).
Babior, B. M., Kipnes, R. S. & Curnutte, J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 52, 741–744 (1973).
Nathan, C. F., Mercer-Smith, J. A., DeSantis, N. M. & Palladino, M. A. Role of oxygen in T cell-mediated cytolysis. J. Immunol. 129, 2164–2171 (1982).
Hunt, T. K., Twomey, P., Zederfeldt, B. & Dunphy, J. E. Respiratory gas tensions and pH in healing wounds. Am. J. Surg. 114, 302–307 (1967).
Berry, L. J. & Mitchell, R. B. Influence of simulated altitude on resistance-susceptibility to S. typhimurium infection in mice. Tex. Rep. Biol. Med. 11, 379–401 (1953).
Ehrlich, R. & Mieszkuc, B. J. Effects of space cabin environment on resistance to infection. I. Effect of 18,000-foot altitude on resistance to respiratory infection. J. Infect. Dis. 110, 278–281 (1962).
Gordon, F. B. & Gillmore, J. D. Parabarosis and experimental infections. 4. Effect of varying O2 tensions on chlamydial infection in mice and cell cultures. Aerosp. Med. 45, 257–262 (1974).
Highman, B. & Altland, P. D. A new method for the production of experimental bacterial endocarditis. Proc. Soc. Exp. Biol. Med. 75, 573–577 (1950).
Meerson, F. Z., Evseev, V. A., Davydova, T. V. & Giber, L. M. Effect of adaptation to altitude hypoxia on the nonspecific immunity indices, production of hemagglutinins and development of adjuvant arthritis in rats. Biull. Eksp. Biol. Med. 89, 12–14 (1980) (in Russian).
Singh, I. et al. Effects of high altitude stay on the incidence of common diseases in man. Int. J. Biometeorol. 21, 93–122 (1977).
Roosevelt, T. S., Ruhmann-Wennhold, A. & Nelson, D. H. A protective effect of glucocorticoids in hypoxic stress. Am. J. Physiol. 223, 30–33 (1972).
Fauci, A. S. & Dale, D. C. The effect of hydrocortisone on the kinetics of normal human lymphocytes. Blood 46, 235–243 (1975).
Zuckerberg, A. L., Goldberg, L. I. & Lederman, H. M. Effects of hypoxia on interleukin-2 mRNA expression by T lymphocytes. Crit. Care Med. 22, 197–203 (1994).
Lederer, J. A., Rodrick, M. L. & Mannick, J. A. The effects of injury on the adaptive immune response. Shock 11, 153–159 (1999).
Confori, L. et al. Hypoxia regulates expression and activity of Kv1.3 channel in T lymphocytes: a possible role in T cell proliferation. J. Immunol. 170, 695–702 (2003).
Robbins, J. R. et al. Hypoxia modulates early events in T cell receptor-mediated activation in human T lymphocytes via Kv1.3 channels. J. Physiol. 564, 131–143 (2005).
Lopez-Barneo, J., Pardal, R. & Ortega-Saenz, P. Cellular mechanism of oxygen sensing. Annu. Rev. Physiol. 63, 259–287 (2001).
Panther, E. et al. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood 101, 3985–3990 (2003).
Yun, J. K. et al. Inflammatory mediators are perpetuated in macrophages resistant to apoptosis induced by hypoxia. Proc. Natl Acad. Sci. USA 94, 13903–13908 (1997).
Scannell, G. et al. Hypoxia induces a human macrophage cell line to release tumor necrosis factor-α and its soluble receptors in vitro. J. Surg. Res. 54, 281–285 (1993).
Ghezzi, P. et al. Hypoxia increases production of interleukin-1 and tumor necrosis factor by human mononuclear cells. Cytokine 3, 189–194 (1991).
Scannell, G. et al. Effects of trauma on leukocyte intercellular adhesion molecule-1, CD11b, and CD18 expressions. J. Trauma 39, 641–644 (1995).
Leeper-Woodford, S. K. & Mills, J. W. Phagocytosis and ATP levels in alveolar macrophages during acute hypoxia. Am. J. Respir. Cell Mol. Biol. 6, 326–334 (1992).
Segal, A. W., Geisow, M., Garcia, R., Harper, A. & Miller, R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature 290, 406–409 (1981).
Semenza, G. L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 15, 551–578 (1999).
Carmeliet, P. et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998). This study showed that HIF1α is responsible for tumour vascularization and growth, as well as for hypoxia-induced apoptosis in tumours.
Koshiba, M., Kojima, H., Huang, S., Apasov, S. & Sitkovsky, M. V. Memory of extracellular adenosine A2A purinergic receptor-mediated signalling in murine T cells. J. Biol. Chem. 272, 25881–25889 (1997).
Huang, S., Koshiba, M., Apasov, S. & Sitkovsky, M. Role of A2A adenosine receptor-mediated signaling in inhibition of T cell activation and expansion. Blood 90, 1600–1610 (1997).
Kung, A. L., Wang, S., Klco, J. M., Kaelin, W. G. & Livingston, D. M. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nature Med. 6, 1335–1340 (2000).
Walmsley, S. R. et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity. J. Exp. Med. 201, 105–115 (2005). The study showed that HIF1α and the hydroxylase FIH1 are involved in the regulation of neutrophil survival during hypoxia through a nuclear factor-κB-mediated mechanism.
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Appelhoff, R. J. et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 279, 38458–38465 (2004).
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001). This study identified an oxygen-dependent prolyl hydroxylase, which modifies HIF1α and thereby facilitates its interaction with VHL and its subsequent degradation.
Baek, J. H. et al. OS-9 interacts with hypoxia-inducible factor 1α and prolyl hydroxylases to promote oxygen-dependent degradation of HIF-1α. Mol. Cell 17, 503–512 (2005).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999). This article described a crucial role for VHL in the regulation of oxygen-dependent HIF1α degradation.
Nakayama, K. et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1α abundance, and modulates physiological responses to hypoxia. Cell 117, 941–952 (2004). The authors of this paper described new members of the HIF1α regulation pathway—the ubiquitin ligases SIAH1A and SIAH2. These target PHD1 and PHD3 for proteasome-mediated degradation under hypoxic conditions.
Wenger, R. H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 16, 1151–1162 (2002).
Maxwell, P. H. et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc. Natl Acad. Sci. USA 94, 8104–8109 (1997).
Lando, D. et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16, 1466–1471 (2002)
Winn, H. R., Rubio, R., Curnish, R. R. & Berne, R. M. Changes in regional cerebral blood flow (rCBF) caused by increase in CSF concentrations of adenosine and 2-chloroadenosine (CHL-ADO). J. Cereb. Blood Flow Metab. 1, S401–S402 (1981).
Decking, U. K., Schlieper, G., Kroll, K. & Schrader, J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ. Res. 81, 154–164 (1997).
Kobayashi, S., Zimmermann, H. & Millhorn, D. E. Chronic hypoxia enhances adenosine release in rat PC12 cells by altering adenosine metabolism and membrane transport. J. Neurochem. 74, 621–632 (2000).
Thompson, L. F. et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 200, 1395–1405 (2004).
Synnestvedt, K. et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110, 993–1002 (2002).
Ohta, A. & Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414, 916–920 (2001). This article provided the first evidence of a crucial and non-redundant role for the hypoxiaA 2A R pathway in protection from excessive inflammation and tissue damage in vivo.
Gomez, G. & Sitkovsky, M. V. Differential requirement for A2A and A3 adenosine receptors for the protective effect of inosine in vivo. Blood 102, 4472–4478 (2003).
Lukashev, D., Ohta, A., Apasov, S. & Sitkovsky, M. Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J. Immunol. 173, 21–24 (2004).
Fredholm, B. B., Ijzerman, A. P., Jacobson, K. A., Klotz, K. -N. & Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 53, 527–552 (2001).
Lukashev, D. E. et al. Analysis of A2A receptor-deficient mice reveals no significant compensatory increases in the expression of A2B, A1, and A3 adenosine receptors in lymphoid organs. Biochem. Pharmacol. 65, 2081–2090 (2003).
Cronstein, B., Daguma, L., Nichols, D., Hutchison, A. & Williams, M. The adenosine/neutrophil paradox resolved: human neutrophils posess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J. Clin. Invest. 85, 1150–1157 (1990).
Lappas, C. M., Rieger, J. M. & Linden, J. A2A adenosine receptor induction inhibits IFN-γ production in murine CD4+ T cells. J. Immunol. 174, 1073–1080 (2005). The authors of this article described that TCR-mediated activation of T cells induces A 2A R expression, which provides a mechanism for limiting T-cell activation and therefore for protecting tissues.
Makino, Y. et al. Hypoxia-inducible factor regulates survival of antigen receptor-driven T cells. J. Immunol. 171, 6534–6540 (2003).
Fujio, Y., Nguyen, T., Wencker, D., Kitsis, R. N. & Walsh, K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101, 660–667 (2000).
Biju, M. P. et al. Vhlh gene deletion induces Hif-1-mediated cell death in thymocytes. Mol. Cell. Biol. 24, 9038–9047 (2004).
Thornton, R. D. et al. Interleukin 1 induces hypoxia-inducible factor 1 in human gingival and synovial fibroblasts. Biochem. J. 350, 307–312 (2000).
Stiehl, D. P., Jelkmann, W., Wenger, R. H. & Hellwig-Burgel, T. Normoxic induction of the hypoxia-inducible factor 1α by insulin and interleukin-1α involves the phosphatidylinositol 3-kinase pathway. FEBS Lett. 512, 157–162 (2002).
Zelzer, E. et al. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 17, 5085–5094 (1998).
Richard, D. E., Berra, E. & Pouyssegur, J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1α in vascular smooth muscle cells. J. Biol. Chem. 275, 26765–26771 (2000).
Mazure, N. M., Chen, E. Y., Laderoute, K. R. & Giaccia, A. J. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood 90, 3322–3331 (1997).
Richard, D. E., Berra, E., Gothie, E., Roux, D. & Pouyssegur, J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1α (HIF-1α) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 274, 32631–32637 (1999).
Wenger, R. H., Rolfs, A., Spielmann, P., Zimmermann, D. R. & Gassmann, M. Mouse hypoxia-inducible factor-1α is encoded by two different mRNA isoforms: expression from a tissue-specific and a housekeeping-type promoter. Blood 91, 3471–3480 (1998).
Iyer, N. V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12, 149–162 (1998). This study showed that HIF1α-deficient embryonic stem cells have impaired proliferation and decreased levels of glycolytic enzymes and VEGF. HIF1α-deficient embryos have lethal developmental defects.
Kong, T., Eltzschig, H. K., Karhausen, J., Colgan, S. P. & Shelley, C. S. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of α2 integrin gene expression. Proc. Natl Acad. Sci. USA 101, 10440–10445 (2004).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, 721–732 (2003).
Hanze, J. et al. RNA interference for HIF-1α inhibits its downstream signalling and affects cellular proliferation. Biochem. Biophys. Res. Commun. 312, 571–577 (2003).
Welsh, S., Williams, R., Kirkpatrick, L., Paine-Murrieta, G. & Powis, G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1α. Mol. Cancer Ther. 3, 233–244 (2004).
Tan, C. et al. Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res. 65, 605–612 (2005).
Berra, E. et al. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J. 22, 4082–4090 (2003).
Metzen, E., Zhou, J., Jelkmann, W., Fandrey, J. & Brune, B. Nitric oxide impairs normoxic degradation of HIF-1α by inhibition of prolyl hydroxylases. Mol. Biol. Cell 14, 3470–3481 (2003).
Hughson, M. D., He, Z., Liu, S., Coleman, J. & Shingleton, W. B. Expression of HIF-1 and ubiquitin in conventional renal cell carcinoma: relationship to mutations of the von Hippel–Lindau tumor suppressor gene. Cancer Genet. Cytogenet. 143, 145–153 (2003).
Bemis, L. et al. Distinct aerobic and hypoxic mechanisms of HIF-α regulation by CSN5. Genes Dev. 18, 739–744 (2004).
Trounce, I. Genetic control of oxidative phosphorylation and experimental models of defects. Hum. Reprod. 15 (Suppl. 2), 18–27 (2000).
Dang, C. V. & Semenza, G. L. Oncogenic alterations of metabolism. Trends Biochem. Sci. 24, 68–72 (1999).
O'Donnell-Tormey, J., Nathan, C. F., Lanks, K., DeBoer, C. J. & de la Harpe, J. Secretion of pyruvate. An antioxidant defense of mammalian cells. J. Exp. Med. 165, 500–514 (1987).
Mongan, P. D. et al. Pyruvate improves redox status and decreases indicators of hepatic apoptosis during hemorrhagic shock in swine. Am. J. Physiol. Heart Circ. Physiol. 283, H1634–H1644 (2002).
Semenza, G. L. & Wang, G. L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 12, 5447–5454 (1992). This seminal paper opened the field of studies on HIF1α.
Semenza, G. L., Roth, P. H., Fang, H. M. & Wang, G. L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23763 (1994). This article established the wide-ranging transcriptional activities of HIF1 α.
Forsythe, J. A. et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16, 4604–4613 (1996).
Metzen, E. et al. Intracellular localisation of human HIF-1 hydroxylases: implications for oxygen sensing. J. Cell Sci. 116, 1319–1326 (2003).
Acknowledgements
This work was supported, in part, by a grant from the National Institutes of Health (United States) to M.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- ACTIVATION-INDUCED CELL DEATH
-
A mechanism of elimination of potentially autoreactive T cells. It occurs through Tcell-receptor-mediated activation, which produces a block in the cell cycle followed by apoptotic death.
- RECOMBINATION-ACTIVATING GENE 2 (RAG2)-DEFICIENT BLASTOCYST-COMPLEMENTATION SYSTEM
-
A gene-targeting technique used for the genetic analysis of lymphocytes. Complementation of RAG2-deficient blastocysts (which cannot produce mature T or B cells) with embryonic stem cells that have a mutation in the gene of interest creates chimeras that have genetically altered T and B cells.
- B1 CELL
-
A cell belonging to the B-cell lineage that mainly populates the peritoneum and secretes polyspecific, low-affinity IgM.
- B2 CELL
-
A conventional B cell. These cells reside in secondary lymphoid organs and secrete antigen-specific antibodies.
- Cre–loxP TECHNOLOGY
-
A method for targeting specific genes by homologous recombination using the bacteriophage P1 recombinase Cre, which recognizes specific loxP sites in target DNA and catalyses recombination between these sites. This results in the excision of the DNA sequence between them.
Rights and permissions
About this article
Cite this article
Sitkovsky, M., Lukashev, D. Regulation of immune cells by local-tissue oxygen tension: HIF1α and adenosine receptors. Nat Rev Immunol 5, 712–721 (2005). https://doi.org/10.1038/nri1685
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1685
This article is cited by
-
In vivo oxygen measurement in cerebrospinal fluid of pigs to determine physiologic and pathophysiologic oxygen values during CNS infections
BMC Neuroscience (2021)
-
The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment
Cancer Cell International (2021)
-
The effect of hypoxia on PD-L1 expression in bladder cancer
BMC Cancer (2021)
-
Mechanisms controlling bacterial infection in myeloid cells under hypoxic conditions
Cellular and Molecular Life Sciences (2021)
-
Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment
Cellular & Molecular Immunology (2020)