Key Points
-
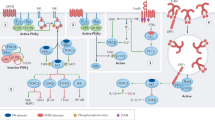
PI3Kδ (phosphoinositide 3-kinase-δ) is a key signal transduction node in cells of the immune system. This kinase complex is acutely activated in B cells and T cells after exposure to antigen and controls many aspects of lymphocyte development and differentiation, in part via the AKT, forkhead box O1 (FOXO1) and mechanistic target of rapamycin (mTOR) pathways.
-
Rare loss-of-function mutations affecting PI3Kδ also cause immunodeficiency and immune-mediated pathologies, including colitis. The PI3Kδ inhibitor idelalisib frequently causes colitis at doses tested in leukaemia and lymphoma trials, possibly due to effects on regulatory T (Treg) cells.
-
Activated PI3Kδ syndrome (APDS; also called PASLI disease) is a newly described primary immunodeficiency caused by hyperactive PI3Kδ signalling and resultant T cell senescence and/or death and impaired antibody responses. APDS is generally characterized by recurrent sinopulmonary infections with structural lung damage, viraemia with herpes family viruses, lymphoproliferative disease and increased risk of B cell malignancies.
-
Patients with APDS1 have a heterozygous mutation in PIK3CD, the gene encoding the p110δ catalytic subunit of PI3Kδ, whereas APDS2 patients have a heterozygous mutation in PIK3R1, the gene encoding the p85α regulatory subunit of PI3Kδ. Both sets of mutations lead to higher intrinsic activity of PI3Kδ.
-
To date, most patients with APDS have been treated with antibody replacement therapies and some have also been treated with the mTOR inhibitor rapamycin. In the future, PI3Kδ inhibitors may be used to treat these patients, possibly as the first example of targeted therapy against a hyperactive mutant kinase in primary immunodeficiency.
Abstract
Primary immunodeficiencies are inherited disorders of the immune system, often caused by the mutation of genes required for lymphocyte development and activation. Recently, several studies have identified gain-of-function mutations in the phosphoinositide 3-kinase (PI3K) genes PIK3CD (which encodes p110δ) and PIK3R1 (which encodes p85α) that cause a combined immunodeficiency syndrome, referred to as activated PI3Kδ syndrome (APDS; also known as p110δ-activating mutation causing senescent T cells, lymphadenopathy and immunodeficiency (PASLI)). Paradoxically, both loss-of-function and gain-of-function mutations that affect these genes lead to immunosuppression, albeit via different mechanisms. Here, we review the roles of PI3Kδ in adaptive immunity, describe the clinical manifestations and mechanisms of disease in APDS and highlight new insights into PI3Kδ gleaned from these patients, as well as implications of these findings for clinical therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
30 September 2016
In Table 1 of the original version published online, the 'C416R' mutation was incorrectly referred to as 'C416K'. The authors and Nature Reviews Immunology apologise for this error. It has been corrected in the print and online versions of the article.
References
Angulo, I. et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science 342, 866–871 (2013).
Lucas, C. L. et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat. Immunol. 15, 88–97 (2014). References 1 and 2 are the first papers showing that activated mutations in PIK3CD cause the PID APDS.
Deau, M. C. et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J. Clin. Invest. 124, 3923–3928 (2014).
Lucas, C. L. et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J. Exp. Med. 211, 2537–2547 (2014). References 3 and 4 are the first papers showing that activating mutations in PIK3R1 cause the PID APDS2.
Elgizouli, M. et al. Activating PI3Kδ mutations in a cohort of 669 patients with primary immunodeficiency. Clin. Exp. Immunol. 183, 221–229 (2016).
Elkaim, E. et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase δ syndrome 2: A cohort study. J. Allergy Clin. Immunol. 138, 210–218 (2016). Clinical and immunological features associated with activating PIK3R1 mutations: a survey of 36 patients.
Coulter, T. I. et al. Clinical spectrum and features of activated PI3-kinase delta syndrome: a large patient cohort study. J. Allergy Clin. Immunol. http://dx.doi.org/10.1016/j.jaci.2016.06.021 (2016). Clinical and immunological features associated with activating PIK3CD mutations: a survey of 53 patients.
Okkenhaug, K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu. Rev. Immunol. 31, 675–704 (2013).
Zhang, K. J., Husami, A., Marsh, R. & Jordan, M. B. Identification of a phosphoinositide 3-kinase (PI-3K) p110δ (PIK3CD) deficient individual. J. Clin. Immunol. 33, 673–674 (2013). The first description of a young male lacking PIK3CD expression.
Conley, M. E. et al. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85α subunit of PI3K. J. Exp. Med. 209, 463–470 (2012). The first description of a young female lacking PIK3R1 expression.
Vanhaesebroeck, B., Whitehead, M. A. & Pineiro, R. Molecules in medicine mini-review: isoforms of PI3K in biology and disease. J. Mol. Med. (Berl.) 94, 5–11 (2016).
Ciraolo, E. et al. Phosphoinositide 3-kinase p110β activity: key role in metabolism and mammary gland cancer but not development. Sci. Signal. 1, ra3 (2008).
Kulkarni, S. et al. PI3Kβ plays a critical role in neutrophil activation by immune complexes. Sci. Signal. 4, ra23 (2011).
Hawkins, P. T. & Stephens, L. R. PI3K signalling in inflammation. Biochim. Biophys. Acta 1851, 882–897 (2015).
Webb, L. M., Vigorito, E., Wymann, M. P., Hirsch, E. & Turner, M. Cutting edge: T cell development requires the combined activities of the p110γ and p110δ catalytic isoforms of phosphatidylinositol 3-kinase. J. Immunol. 175, 2783–2787 (2005).
Fruman, D. A. Regulatory subunits of class IA PI3K. Curr. Top. Microbiol. Immunol. 346, 225–244 (2010).
Burke, J. E. & Williams, R. L. Synergy in activating class I PI3Ks. Trends Biochem. Sci. 40, 88–100 (2015). An excellent review on the structure and function of PI3K, explaining how individual mutations in PIK3R1 or PIK3CD lead to PI3K δ activation.
Fritsch, R. et al. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 153, 1050–1063 (2013).
Suzuki, H. et al. Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science 283, 390–392 (1999).
Fruman, D. A. et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science 283, 393–397 (1999).
Clayton, E. et al. A crucial role for the p110δ subunit of phosphatidylinositol 3-kinase in B cell development and activation. J. Exp. Med. 196, 753–763 (2002).
Okkenhaug, K. et al. Impaired B and T cell antigen receptor signaling in p110δ PI3-kinase mutant mice. Science 297, 1031–1034 (2002).
Jou, S. T. et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110δ in signaling by the B-cell receptor complex. Mol. Cell. Biol. 22, 8580–8591 (2002).
Ramadani, F. et al. The PI3K isoforms p110α and p110δ are essential for pre-B cell receptor signaling and B cell development. Sci. Signal. 3, ra60 (2010).
Zhang, T. T. et al. Genetic or pharmaceutical blockade of p110δ phosphoinositide 3-kinase enhances IgE production. J. Allergy Clin. Immunol. 122, 811–819 (2008).
Janas, M. L. et al. The effect of deleting p110δ on the phenotype and function of PTEN-deficient B cells. J. Immunol. 180, 739–746 (2008).
Rolf, J. et al. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J. Immunol. 185, 4042–4052 (2010).
Durand, C. A. et al. Phosphoinositide 3-kinase p110δ regulates natural antibody production, marginal zone and B-1 B cell function, and autoantibody responses. J. Immunol. 183, 5673–5684 (2009).
Alkhatib, A. et al. FoxO1 induces Ikaros splicing to promote immunoglobulin gene recombination. J. Exp. Med. 209, 395–406 (2012).
Dengler, H. S. et al. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat. Immunol. 9, 1388–1398 (2008).
Llorian, M., Stamataki, Z., Hill, S., Turner, M. & Martensson, I. L. The PI3K p110δ is required for down-regulation of RAG expression in immature B cells. J. Immunol. 178, 1981–1985 (2007).
Shojaee, S. et al. PTEN opposes negative selection and enables oncogenic transformation of pre-B cells. Nat. Med. 22, 379–387 (2016).
Kinoshita, K. & Honjo, T. Unique and unprecedented recombination mechanisms in class switching. Curr. Opin. Immunol. 12, 195–198 (2000).
Suzuki, A. et al. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J. Exp. Med. 197, 657–667 (2003).
Omori, S. A. et al. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity 25, 545–557 (2006).
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol. 30, 429–457 (2012).
Dominguez-Sola, D. et al. The FOXO1 transcription factor instructs the germinal center dark zone program. Immunity 43, 1064–1074 (2015).
Sander, S. et al. PI3 Kinase and FOXO1 transcription factor activity differentially control B cells in the germinal center light and dark zones. Immunity 43, 1075–1086 (2015).
Gigoux, M. et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc. Natl Acad. Sci. USA 106, 20371–20376 (2009).
Okkenhaug, K. et al. The p110δ isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J. Immunol. 177, 5122–5128 (2006).
Soond, D. R. et al. PI3K p110δ regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood 115, 2203–2213 (2010).
Kurebayashi, Y. et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORγ. Cell Rep. 1, 360–373 (2012).
Ouyang, W. et al. Novel Foxo1-dependent transcriptional programs control Treg cell function. Nature 491, 554–559 (2012).
Delgoffe, G. M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 (2011).
Nashed, B. F. et al. Role of the phosphoinositide 3-kinase p110δ in generation of type 2 cytokine responses and allergic airway inflammation. Eur. J. Immunol. 37, 416–424 (2007).
Haylock-Jacobs, S. et al. PI3Kδ drives the pathogenesis of experimental autoimmune encephalomyelitis by inhibiting effector T cell apoptosis and promoting Th17 differentiation. J. Autoimmun. 36, 278–287 (2011).
Liu, D. et al. The p110δ isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J. Immunol. 183, 1921–1933 (2009).
Patton, D. T. et al. Cutting edge: the phosphoinositide 3-kinase p110δ is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J. Immunol. 177, 6598–6602 (2006).
Coutre, S. E. et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk. Lymphoma 56, 2779–2786 (2015).
O'Brien, S. M. et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood 126, 2686–2694 (2015).
Ali, K. et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 510, 407–411 (2014).
Aksoy, E. et al. The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat. Immunol. 13, 1045–1054 (2012).
Pearce, V. Q., Bouabe, H., MacQueen, A. R., Carbonaro, V. & Okkenhaug, K. PI3Kδ regulates the magnitude of CD8+ T cell responses after challenge with Listeria monocytogenes. J. Immunol. 195, 3206–3217 (2015).
Putz, E. M. et al. PI3Kδ is essential for tumor clearance mediated by cytotoxic T lymphocytes. PLoS ONE 7, e40852 (2012).
Sinclair, L. V. et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat. Immunol. 9, 513–521 (2008).
Gracias, D. T. et al. Phosphatidylinositol 3-kinase p110δ isoform regulates CD8+ T cell responses during acute viral and intracellular bacterial infections. J. Immunol. 196, 1186–1198 (2016).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Suzuki, A. et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14, 523–534 (2001).
Borlado, L. R. et al. Increased phosphoinositide 3-kinase activity induces a lymphoproliferative disorder and contributes to tumor generation in vivo. FASEB J. 14, 895–903 (2000).
Soond, D. R. et al. Pten loss in CD4 T cells enhances their helper function but does not lead to autoimmunity or lymphoma. J. Immunol. 188, 5935–5943 (2012).
Kim, M. V., Ouyang, W., Liao, W., Zhang, M. Q. & Li, M. O. The transcription factor Foxo1 controls central-memory CD8+ T cell responses to infection. Immunity 39, 286–297 (2013).
Condliffe, A. M. et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood 106, 1432–1440 (2005).
Jou, S. T. et al. Identification of variations in the human phosphoinositide 3-kinase p110δ gene in children with primary B-cell immunodeficiency of unknown aetiology. Int. J. Immunogenet. 33, 361–369 (2006).
Chiriaco, M. et al. A case of APDS patient: defects in maturation and function and decreased in vitro anti-mycobacterial activity in the myeloid compartment. Clin. Immunol. http://dx.doi.org/10.1016/j.clim.2015.12.008 (2015).
Crank, M. C. et al. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J. Clin. Immunol. 34, 272–276 (2014).
Hartman, H. N. et al. Gain of function mutations of PIK3CD as a cause of primary sclerosing cholangitis. J. Clin. Immunol. 35, 11–14 (2014).
Kracker, S. et al. Occurrence of B-cell lymphomas in patients with activated phosphoinositide 3-kinase δ syndrome. J. Allergy Clin. Immunol. 134, 233–236 (2014).
Rae, W. et al. Precision treatment with sirolimus in a case of activated phosphoinositide 3-kinase δ syndrome. Clin. Immunol. http://dx.doi.org/10.1016/j.clim.2016.07.017 (2016).
Tsujita, Y. et al. Phosphatase and tensin homolog (PTEN) mutation can cause activated phosphatidylinositol 3-kinase δ syndrome-like immunodeficiency. J. Allergy Clin. Immunol. http://dx.doi.org/10.1016/j.jaci.2016.03.055 (2016).
Kuhlen, M. et al. De novo PIK3R1 gain-of-function with recurrent sinopulmonary infections, long-lasting chronic CMV-lymphadenitis and microcephaly. Clin. Immunol. 162, 27–30 (2016).
Lougaris, V. et al. Altered germinal center reaction and abnormal B cell peripheral maturation in PI3KR1-mutated patients presenting with HIGM-like phenotype. Clin. Immunol. 159, 33–36 (2015).
Petrovski, S. et al. dominant splice site mutations in PIK3R1 cause hyper IgM syndrome, lymphadenopathy and short stature. J. Clin. Immunol. 36, 462–471 (2016).
Olbrich, P. et al. Activated PI3Kδ syndrome type 2: Two patients, a novel mutation and review of the literature. Pediatr. Allergy Immunol. http://dx.doi.org/10.1111/pai.12585 (2016).
Driessen, G. J. et al. Increased PI3K/Akt activity and deregulated humoral immune response in human PTEN deficiency. J. Allergy Clin. Immunol. http://dx.doi.org/10.1016/j.jaci.2016.07.010 (2016).
Browning, M. J., Chandra, A., Carbonaro, V., Okkenhaug, K. & Barwell, J. Cowden's syndrome with immunodeficiency. J. Med. Genet. 52, 856–859 (2015).
Jaiswal, B. S. et al. Somatic mutations in p85α promote tumorigenesis through class IA PI3K activation. Cancer Cell 16, 463–474 (2009).
Urick, M. E. et al. PIK3R1 (p85α) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 71, 4061–4067 (2011).
Di Fonte, R., Baronio, M., Plebani, A., Lougaris, V. & Fousteri, G. Reduced germinal center follicular helper T cells but normal follicular regulatory T cells in the tonsils of a patient with a mutation in the PI3KR1 gene. Clin. Immunol. 164, 43–44 (2016).
Sapey, E. et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood 123, 239–248 (2014).
Brenchley, J. M. et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101, 2711–2720 (2003).
Hathcock, K. S., Jeffrey Chiang, Y. & Hodes, R. J. In vivo regulation of telomerase activity and telomere length. Immunol. Rev. 205, 104–113 (2005).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Hukelmann, J. L. et al. The cytotoxic T cell proteome and its shaping by the kinase mTOR. Nat. Immunol. 17, 104–112 (2016).
Furman, R. R. et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 370, 997–1007 (2014).
Gopal, A. K. et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 370, 1008–1018 (2014).
Boisson, B., Quartier, P. & Casanova, J. L. Immunological loss-of-function due to genetic gain-of-function in humans: autosomal dominance of the third kind. Curr. Opin. Immunol. 32, 90–105 (2015).
Thauvin-Robinet, C. et al. PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am. J. Hum. Genet. 93, 141–149 (2013).
Schroeder, C. et al. PIK3R1 mutations in SHORT syndrome. Clin. Genet. 86, 292–294 (2014).
Dyment, D. A. et al. Mutations in PIK3R1 cause SHORT syndrome. Am. J. Hum. Genet. 93, 158–166 (2013).
Chudasama, K. K. et al. SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling. Am. J. Hum. Genet. 93, 150–157 (2013).
Acknowledgements
The authors thank R. Kissinger at Visual Medical Arts, Research and Technologies Branch, National Institute of Allergy and Infectious Diseases (NIAID), US National Institutes of Health (NIH) for artistic contributions to initial drafts of figures 1–4. C.L.L. was supported by the Intramural Research Program of NIAID, NIH and is now supported by a K99/R00 award from the National Heart, Lung and Blood Institute (NHLBI), NIH and Yale University. A.C. and S.N. are supported by fellowships from the Wellcome Trust, UK. S.N., A.M.C. and K.O. are recipients of a programme grant MR/M012328/2 from the UK medical Research Council and GlaxoSmithKline to investigate APDS. S.N. is also supported by the EU FP7 collaborative grant 261441 and the UK National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre. A.M.C. also received funding from the British Lung Foundation (RG14-1). Work in the K.O. laboratory is also supported by grants from the Biotechnology and Biological Sciences Research Council (BBSRC: BBS/E/B/000C0407, BBS/E/B/000C0409) and from the Wellcome Trust (095691/Z/11/Z). We are grateful to the many colleagues who have contributed to our understanding of APDS. The authors apologize to those whose contributions could not be cited owing to space constraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.L.L. collaborates with Novartis. A.C., S.N., A.M.C. and K.O. collaborate with and receive research funding from GlaxoSmithKline. K.O. has received consultancy or speaker fees from Karus Therapeutics, Merck, Gilead and Incyte.
Related links
Glossary
- Activated phosphoinositide 3-kinase-δ (PI3Kδ) syndrome
-
(APDS).The term APDS encompasses two syndromes: APDS1 (also known as PASLI-CD), which results from a mutation in the PIK3CD gene that leads to the hyperactivation of the p110δ subunit of PI3Kδ; and APDS2 (also known as PASLI-R1), which results from a splice mutation in PIK3R1 that leads to exon skipping and produces a truncated p85α protein with reduced inhibition of p110δ.
- Primary immunodeficiency
-
(PID). An inherited disorder of the immune system that leads to recurrent infections and/or immune dysregulation. Currently there are around 84,000 patients diagnosed worldwide with PID.
- Hypogammaglobulinaemia
-
An immune disorder characterized by low serum IgG levels.
- Immune complexes
-
Complexes of antigen bound to antibody and, sometimes, components of the complement system. The levels of immune complexes are increased in many autoimmune disorders, in which they become deposited in tissues and cause tissue damage.
- Class-switch recombination
-
(CSR).The process by which B cells rearrange their DNA to switch from expressing IgM (or another class of immunoglobulin) to expressing a different immunoglobulin heavy-chain constant region, thereby producing antibody with different effector functions.
- T cell-independent (TI) antibody response
-
An antibody response to polymeric antigens, such as polysaccharides and lipids, that does not require T cell help.
- Activation-induced cytidine deaminase
-
(AID). An enzyme that is required for two crucial events in the germinal centre: somatic hypermutation and class-switch recombination.
- Somatic hypermutation
-
(SHM). A unique mutation mechanism that is targeted to the variable regions of rearranged immunoglobulin gene segments. Combined with selection for B cells thatproduce high-affinity antibody, SHM leads to affinity maturation of B cells in germinal centres.
- Germinal centre reaction
-
Germinal centres are specialized structures within spleens and lymph nodes where B cells present antigen to T cells and, in return, are selected to undergo class-switch recombination and somatic hypermutation.
- Follicular helper T cells
-
(TFH cells). CD4+ T helper cells that are essential for the induction of class switching in the germinal centres of secondary follicles during antibody responses to T cell-dependent antigens.
- Transitional B cells
-
Immature B cells that have left the bone marrow for the spleen and are precursors of follicular B cells, marginal zone B cells and B1 B cells.
- Senescence
-
A state in which a cell fails to progress through the cell cycle owing to activation of the DNA damage response, which can occur upon extreme shortening of telomeres.
Rights and permissions
About this article
Cite this article
Lucas, C., Chandra, A., Nejentsev, S. et al. PI3Kδ and primary immunodeficiencies. Nat Rev Immunol 16, 702–714 (2016). https://doi.org/10.1038/nri.2016.93
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2016.93
This article is cited by
-
Activated phosphoinositide 3-kinase δ syndrome caused by PIK3CD mutations: expanding the phenotype
Pediatric Rheumatology (2024)
-
Modulating the PI3K Signalling Pathway in Activated PI3K Delta Syndrome: a Clinical Perspective
Journal of Clinical Immunology (2024)
-
Two cases of successful sirolimus treatment for patients with activated phosphoinositide 3-kinase δ syndrome 1
Allergy, Asthma & Clinical Immunology (2023)
-
Genome-wide allele frequency studies in Pacific oyster families identify candidate genes for tolerance to ostreid herpesvirus 1 (OsHV-1)
BMC Genomics (2023)
-
Epstein–Barr virus-associated B-cell lymphoproliferative disorder meeting the definition of CAEBV B cell disease: a case report
BMC Infectious Diseases (2023)