Key Points
-
Although the platelet was once considered to be merely a passive participant in the coagulation cascade, it is now recognized as an active synthesizer of humoral factors that potentiate both clot formation and inflammation.
-
For several decades, antiplatelet therapy centered on the thromboxane pathway and its inhibition by aspirin, and aspirin remains the background template therapy for acute ischaemic syndromes, as well as for secondary prevention.
-
However, despite the wealth of data that support the use of aspirin, and indeed the data that suggest it should be more widely prescribed, aspirin remains a suboptimal antiplatelet agent, as evidenced clinically by the fact that even patients who are taking aspirin may nevertheless sustain a thrombotic event.
-
Drugs have been developed to improve on the template of aspirin therapy; the glycoprotein IIb/IIIa inhibitors and the thienopyridines have been extensively studied.
-
The glycoprotein IIb/IIIa receptor is an integrin found in high concentration on the platelet membrane, and is often referred to as the final common pathway of platelet aggregation. The evidence supporting the utility of intravenous GPIIb/IIIa inhibition in percutaneous coronary intervention is overwhelming.
-
In contrast to the favourable data obtained for the intravenous GPIIb/IIIa inhibitors, results with oral GPIIb/IIIa inhibitors have been disappointing. This showed that our understanding of GPIIb/IIIa inhibition was not as deep as believed. Indeed, it could be that this class of agents has a potential dark side, perhaps due to sub-therapeutic levels of drug leading to platelet activation and/or inflammation.
-
So, although the intravenous GPIIb/IIIa inhibitors represent a major therapeutic advance, it is unlikely that there will be any further development or refinement of these agents.
-
Clopidogrel, and its predecessor ticlopidine, are thienopyridines that act by antagonising the receptors for adenosine 5′-diphosphate, which have key roles in platelet activation, shape change and aggregation.
-
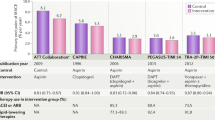
Clopidogrel represents a major advance in antiplatelet therapy. In the CAPRIE trial, an 8.7% relative risk reduction in vascular death, ischaemic stroke or myocardial infarction was found with clopidogrel versus aspirin. Subset analyses of the CAPRIE study have demonstrated that the benefits of clopidogrel over aspirin are amplified in high-risk patients.
-
Although it is clear that clopidogrel is more effective than aspirin alone, dual antiplatelet pathway inhibition is more than additive in preventing thrombus formation. Several trials are planned or are ongoing with dual antiplatelet therapy.
-
Greater understanding of platelet biology is revealing novel, and potentially more precise, targets for the therapeutic modulation of platelet function. Several potential future targets for antiplatelet drugs are discussed, including: P2Y1 receptor, P2Y12 receptor, P2Y1 and P2Y12 receptors simultaneously, CD40–CD40 ligand system, RANTES, P-selectin, CD39/ATPDase, GP Ib-V-IX complex - von Willebrand factor, protease-activated receptors (PARs), pepducins, platelet leptin receptor, Arp2/3 complex, and Growth-arrest specific gene 6 (Gas6).
Abstract
Over the past decade, the platelet has emerged as a pivotal entity in cardiovascular diseases. Indeed, the 'preeminence of the platelet' has spawned a variety of drugs that have been shown in large-scale randomized trials to improve patient outcomes in acute coronary syndromes and percutaneous revascularization procedures. Although the platelet was initially viewed only as a bystander in haemostasis, it is now evident that the platelet is in fact a key mediator of thrombosis as well as of inflammation. New insights at the cellular and genomic levels will probably generate novel drugs to inhibit platelet function more effectively and safely than previously possible.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vane, J. R., Flower, R. J. & Botting, R. M. History of aspirin and its mechanism of action. Stroke 21, IV12–23 (1990).
Topol, E. J. & Serruys, P. W. Frontiers in interventional cardiology. Circulation 98, 1802–1820 (1998).
Bhatt, D. L. & Topol, E. J. Antiplatelet and anticoagulant therapy in the secondary prevention of ischemic heart disease. Med. Clin. North Am. 84, 163–179 (2000). An overview of the clinical data regarding both antiplatelet and anticoagulant drugs.
Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ 308, 81–106 (1994).
Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 2, 349–360 (1988).
Antithrombotic Trialists' Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324, 71–86 (2002). This paper provides an updated overview of the trials of antiplatelet therapy, particularly aspirin.
Sanmuganathan, P. S., Ghahramani, P., Jackson, P. R., Wallis, E. J. & Ramsay, L. E. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 85, 265–271 (2001). This meta-analysis summarizes the data regarding aspirin for primary prevention.
U.S. Preventive Services Task Force Aspirin for the primary prevention of cardiovascular events: recommendation and rationale. Ann. Intern. Med. 136, 157–160 (2002).
Hayden, M., Pignone, M., Phillips, C. & Mulrow, C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U. S. Preventive Services Task Force. Ann. Intern. Med. 136, 161–172 (2002).
Gum, P. A., Thamilarasan, M., Watanabe, J., Blackstone, E. H. & Lauer, M. S. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: A propensity analysis. JAMA 286, 1187–1194 (2001).
Yusuf, S. et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345, 494–502 (2001). This paper presents the main findings of the CURE study of aspirin plus clopidogrel versus aspirin alone in patients with acute coronary syndromes.
Taylor, D. W. et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomized controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 353, 2179–2184 (1999).
Bhatt, D. L., Kapadia, S. R., Yadav, J. S. & Topol, E. J. Update on clinical trials of antiplatelet therapy for cerebrovascular diseases. Cerebrovasc. Dis. 10 (Suppl S5), 34–40 (2000).
The Ridogrel Versus Aspirin Patency Trial Randomized trial of ridogrel, a combined thromboxane A2 synthase inhibitor and thromboxane A2/prostaglandin endoperoxide receptor antagonist, versus aspirin as adjunct to thrombolysis in patients with acute myocardial infarction. The Ridogrel Versus Aspirin Patency Trial (RAPT). Circulation 89, 588–595 (1994).
Michaux, C. et al. Terbogrel, a dual-acting agent for thromboxane receptor antagonism and thromboxane synthase inhibition. Acta Crystallogr. C 56, 1265–1266 (2000).
Soyka, R., Guth, B. D., Weisenberger, H. M., Luger, P. & Muller, T. H. Guanidine derivatives as combined thromboxane A2 receptor antagonists and synthase inhibitors. J. Med. Chem. 42, 1235–1249 (1999).
Langleben, D. et al. Effects of the thromboxane synthetase inhibitor and receptor antagonist terbogrel in patients with primary pulmonary hypertension. Am. Heart. J. 143, E4 (2002).
Napoli, C. et al. Chronic treatment with nitric oxide-releasing aspirin reduces plasma low-density lipoprotein oxidation and oxidative stress, arterial oxidation-specific epitopes, and atherogenesis in hypercholesterolemic mice. Proc. Natl Acad. Sci. USA 99, 12467–12470 (2002).
Napoli, C. et al. Efficacy and age-related effects of nitric oxide-releasing aspirin on experimental restenosis. Proc. Natl Acad. Sci. USA 99, 1689–1694 (2002).
Napoli, C. et al. Effects of nitric oxide-releasing aspirin versus aspirin on restenosis in hypercholesterolemic mice. Proc. Natl Acad. Sci. USA 98, 2860–2864 (2001).
Califf, R. M. et al. Underuse of aspirin in a referral population with documented coronary artery disease. Am. J. Cardiol. 89, 653–661 (2002).
Alexander, J. H. et al. Prior aspirin use predicts worse outcomes in patients with non-ST- elevation acute coronary syndromes. PURSUIT Investigators. Platelet IIb/IIIa in Unstable angina: Receptor Suppression Using Integrilin Therapy. Am. J. Cardiol. 83, 1147–1151 (1999).
Gum, P. A. et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am. J. Cardiol. 88, 230–235 (2001).
Gum, P. A. et al. Clinical consequences of aspirin resistance. J. Am. Coll. Cardiol (in the press).
Eikelboom, J. W. et al. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation 105, 1650–1655 (2002).
Ridker, P. M., Hennekens, C. H., Schmitz, C., Stampfer, M. J. & Lindpaintner, K. PIA1/A2 polymorphism of platelet glycoprotein IIIa and risks of myocardial infarction, stroke, and venous thrombosis. Lancet 349, 385–388 (1997).
Szczeklik, A., Undas, A., Sanak, M., Frolow, M. & Wegrzyn, W. Relationship between bleeding time, aspirin and the PlA1/A2 polymorphism of platelet glycoprotein IIIa. Br. J. Haematol. 110, 965–967 (2000).
Andrioli, G. et al. Defective platelet response to arachidonic acid and thromboxane A(2) in subjects with Pl(A2) polymorphism of β(3) subunit (glycoprotein IIIa). Br. J. Haematol. 110, 911–918 (2000).
Michelson, A. D. et al. Platelet GP IIIa Pl(A) polymorphisms display different sensitivities to agonists. Circulation 101, 1013–1018 (2000).
Undas, A., Brummel, K., Musial, J., Mann, K. G. & Szczeklik, A. Pl(A2) polymorphism of β(3) integrins is associated with enhanced thrombin generation and impaired antithrombotic action of aspirin at the site of microvascular injury. Circulation 104, 2666–2672 (2001).
Topol, E. J. & Quinn, M. J. Common variations in platelet glycoproteins: pharmacogenomic implications. Pharmacogenomics 2, 341–352 (2001).
Weber, A. A., Zimmermann, K. C., Meyer-Kirchrath, J. & Schror, K. Cyclooxygenase-2 in human platelets as a possible factor in aspirin resistance. Lancet 353, 900 (1999).
Topol, E. J., Byzova, T. V. & Plow, E. F. Platelet GPIIb-IIIa blockers. Lancet 353, 227–231 (1999).
Kleiman, N. S. et al. Differential inhibition of platelet aggregation induced by adenosine diphosphate or a thrombin receptor-activating peptide in patients treated with bolus chimeric 7E3 Fab: implications for inhibition of the internal pool of GPIIb/IIIa receptors. J. Am. Coll. Cardiol. 26, 1665–1671 (1995).
Nurden, P. et al. Labeling of the internal pool of GP IIb-IIIa in platelets by c7E3 Fab fragments (abciximab): flow and endocytic mechanisms contribute to the transport. Blood 93, 1622–1633 (1999).
Gawaz, M. et al. Incomplete inhibition of platelet aggregation and glycoprotein IIb-IIIa receptor blockade by abciximab: importance of internal pool of glycoprotein IIb-IIIa receptors. Thromb. Haemost. 83, 915–922 (2000).
Quinn, M. J., Murphy, R. T., Dooley, M., Foley, J. B. & Fitzgerald, D. J. Occupancy of the internal and external pools of glycoprotein IIb/IIIa following abciximab bolus and infusion. J. Pharmacol. Exp. Ther. 297, 496–500 (2001).
Bhatt, D. L. & Topol, E. J. Current role of platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. JAMA 284, 1549–1558 (2000). An overview of the glycoprotein IIb/IIIa inhibitors, particularly their role in acute coronary syndromes and percutaneous coronary intervention.
Topol, E. J. et al. Multi-year follow-up of abciximab therapy in three randomized, placebo- controlled trials of percutaneous coronary revascularization. Am. J. Med. 113, 1–6 (2002).
O'Shea, J. C. et al. Platelet glycoprotein IIb/IIIa integrin blockade with eptifibatide in coronary stent intervention: the ESPRIT trial: a randomized controlled trial. JAMA 285, 2468–2473 (2001).
Topol, E. J. et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N. Engl. J. Med. 344, 1888–1894 (2001). The first and largest head-to-head comparison of intravenous glycoprotein IIb/IIIa inhibitors.
Chew, D. P. & Bhatt, D. L. Optimizing glycoprotein IIb/IIIa inhibition: lessons from recent randomized controlled trials. Intern. Med. J. 32, 338–345 (2002).
Bhatt, D. L. & Topol, E. J. in Acute Coronary Syndromes (ed. Topol, E. J.) 79–110 (Marcel Dekker, New York, 2000).
Hamm, C. W. et al. Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) Study Investigators. N. Engl. J. Med. 340, 1623–1629 (1999).
Hamm, C. W., Heeschen, C., Goldmann, B. U. & White, H. D. Benefit of Tirofiban in High-Risk Patients with Unstable Angina Identified by Troponins in the PRISM Trial. Circulation 100, I–775 (1999).
Bhatt, D. L. et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J. Am. Coll. Cardiol. 35, 922–928 (2000).
Roffi, M. et al. Platelet glycoprotein IIb/IIIa inhibitors reduce mortality in diabetic patients with non-ST-segment-elevation acute coronary syndromes. Circulation 104, 2767–2771 (2001).
Tschoepe, D., Driesch, E., Schwippert, B. & Lampeter, E. F. Activated platelets in subjects at increased risk of IDDM. DENIS Study Group. Deutsche Nikotinamid Interventionsstudie. Diabetologia 40, 573–577 (1997).
Tschoepe, D., Rauch, U. & Schwippert, B. Platelet-leukocyte-cross-talk in diabetes mellitus. Horm. Metab. Res. 29, 631–635 (1997).
Chew, D. P. & Bhatt, D. L. Oral glycoprotein IIb/IIIa antagonists in coronary artery disease. Curr. Cardiol. Rep. 3, 63–71 (2001).
Chew, D. P., Bhatt, D. L., Sapp, S. & Topol, E. J. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: A meta-analysis of phase III multicenter randomized trials. Circulation 103, 201–206 (2001). The initial report that oral glycoprotein IIb/IIIa inhibitors increase the rate of mortality.
Simoons, M. L. Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trial. Lancet 357, 1915–1924 (2001).
Steinhubl, S. R. et al. Point-of-care measured platelet inhibition correlates with a reduced risk of an adverse cardiac event after percutaneous coronary intervention: results of the GOLD (AU-Assessing Ultegra) multicenter study. Circulation 103, 2572–2578 (2001).
Cox, D. et al. Evidence of platelet activation during treatment with a GPIIb/IIIa antagonist in patients presenting with acute coronary syndromes. J. Am. Coll. Cardiol. 36, 1514–1519 (2000).
Quinn, M. J., Plow, E. F. & Topol, E. J. Platelet glycoprotein IIb/IIIa inhibitors: recognition of a two-edged sword? Circulation 106, 379–385 (2002).
O'Connor, F. F. et al. Genetic variation in glycoprotein IIb/IIIa (GPIIb/IIIa) as a determinant of the responses to an oral GPIIb/IIIa antagonist in patients with unstable coronary syndromes. Blood 98, 3256–3260 (2001).
Schneider, D. J., Taatjes, D. J. & Sobel, B. E. Paradoxical inhibition of fibrinogen binding and potentiation of α-granule release by specific types of inhibitors of glycoprotein IIb- IIIa. Cardiovasc Res 45, 437–446 (2000).
Klinkhardt, U., Graff, J. & Harder, S. Clopidogrel, but not abciximab, reduces platelet leukocyte conjugates and P-selectin expression in a human ex vivo in vitro model. Clin. Pharmacol. Ther. 71, 176–185 (2002).
Lincoff, A. M. et al. Abciximab suppresses the rise in levels of circulating inflammatory markers after percutaneous coronary revascularization. Circulation 104, 163–167 (2001).
Steinhubl, S. R. Assessing the optimal level of platelet inhibition with GPIIb/IIIa inhibitors in patients undergoing coronary intervention. Rationale and design of the GOLD study. J. Thromb. Thrombolysis 9, 199–205 (2000).
Adderley, S. R. & Fitzgerald, D. J. Glycoprotein IIb/IIIa antagonists induce apoptosis in rat cardiomyocytes by caspase-3 activation. J. Biol. Chem. 275, 5760–5766 (2000).
Newby, L. K. et al. Benefit of glycoprotein IIb/IIIa inhibition in patients with acute coronary syndromes and troponin t-positive status: the paragon-B troponin T substudy. Circulation 103, 2891–2896 (2001).
Bhatt, D. L. The CRUSADE Registry of high risk acute coronary syndrome patients. Circulation 106, II–494 (2002).
Bhatt, D. L. et al. Safety of concomitant therapy with eptifibatide and enoxaparin in patients undergoing percutaneous coronary intervention – Results of the CRUISE study. J. Am. Coll. Cardiol. (in the press).
Antman, E. M. et al. Enoxaparin as adjunctive antithrombin therapy for ST-elevation myocardial infarction: results of the ENTIRE-Thrombolysis in Myocardial Infarction (TIMI) 23 Trial. Circulation 105, 1642–1649 (2002).
Topol, E. J. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet 357, 1905–1914 (2001).
The Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarction. Lancet 358, 605–613 (2001).
Janzon, L. The STIMS trial: the ticlopidine experience and its clinical applications. Swedish Ticlopidine Multicenter Study. Vasc. Med. 1, 141–143 (1996).
Balsano, F. et al. Antiplatelet treatment with ticlopidine in unstable angina. A controlled multicenter clinical trial. The Studio della Ticlopidina nell'Angina Instabile Group. Circulation 82, 17–26 (1990).
Chevigne, M., David, J. L., Rigo, P. & Limet, R. Effect of ticlopidine on saphenous vein bypass patency rates: a double- blind study. Ann. Thorac. Surg. 37, 371–378 (1984).
Limet, R., David, J. L., Magotteaux, P., Larock, M. P. & Rigo, P. Prevention of aorta-coronary bypass graft occlusion. Beneficial effect of ticlopidine on early and late patency rates of venous coronary bypass grafts: a double-blind study. J. Thorac. Cardiovasc. Surg. 94, 773–783 (1987).
Becquemin, J. P. Effect of ticlopidine on the long-term patency of saphenous-vein bypass grafts in the legs. Etude de la Ticlopidine apres Pontage Femoro- Poplite and the Association Universitaire de Recherche en Chirurgie. N. Engl. J. Med. 337, 1726–1731 (1997).
Hass, W. K. et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N. Engl. J. Med. 321, 501–507 (1989).
Gent, M. et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. Lancet 1, 1215–1220 (1989).
Steinhubl, S. R., Tan, W. A., Foody, J. M. & Topol, E. J. Incidence and clinical course of thrombotic thrombocytopenic purpura due to ticlopidine following coronary stenting. EPISTENT Investigators. Evaluation of Platelet IIb/IIIa Inhibitor for Stenting. JAMA 281, 806–810 (1999).
Zheng, X., Majerus, E. M. & Sadler, J. E. ADAMTS13 and TTP. Curr. Opin. Hematol. 9, 389–394 (2002).
Sadler, J. E. A new name in thrombosis, ADAMTS13. Proc. Natl Acad. Sci. USA 99, 11552–11554 (2002).
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 348, 1329–1339 (1996). The landmark study which demonstrated the superiority of clopidogrel over aspirin.
Bhatt, D. L., Hirsch, A. T., Ringleb, P. A., Hacke, W. & Topol, E. J. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. Am. Heart J. 140, 67–73 (2000).
Bhatt, D. L. et al. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 103, 363–368 (2001).
Bhatt, D. et al. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am. J. Cardiol. 90, 625–628 (2002). A subset analysis of the CAPRIE study which demonstrated particular benefit of antiplatelet therapy with clopidogrel in diabetic patients.
Ringleb, P. A. et al. The benefit of clopidogrel over aspirin is amplified in high–risk subgroups of patients with a prior history of ischemic events. Stroke (in the press).
Herbert, J. M. et al. The antiaggregating and antithrombotic activity of clopidogrel is potentiated by aspirin in several experimental models in the rabbit. Thromb. Haemost. 80, 512–518 (1998).
Makkar, R. R. et al. Effects of clopidogrel, aspirin and combined therapy in a porcine ex vivo model of high-shear induced stent thrombosis. Eur. Heart. J. 19, 1538–1546 (1998).
Moshfegh, K. et al. Antiplatelet effects of clopidogrel compared with aspirin after myocardial infarction: enhanced inhibitory effects of combination therapy. J. Am. Coll. Cardiol. 36, 699–705 (2000).
Rupprecht, H. J. et al. Comparison of antiplatelet effects of aspirin, ticlopidine, or their combination after stent implantation. Circulation 97, 1046–1052 (1998).
Bertrand, M. E. et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirin and ticlopidine (FANTASTIC) study. Circulation 98, 1597–1603 (1998).
Leon, M. B. et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N. Engl. J. Med. 339, 1665–1671 (1998).
Bhatt, D. L. et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J. Am. Coll. Cardiol. 39, 9–14 (2002). The study which established that clopidogrel should be substituted for ticlopidine in patients receiving coronary stenting.
Bertrand, M. E., Rupprecht, H. J., Urban, P., Gershlick, A. H. & Investigators, f. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation 102, 624–629 (2000).
Yadav, J. S. et al. Elective stenting of the extracranial carotid arteries. Circulation 95, 376–381 (1997).
Bhatt, D. L. et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J. Invasive. Cardiol. 13, 767–771 (2001).
Mehta, S. R. et al. Effects of pretreatment with clopidogrel and aspirin followed by long- term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 358, 527–533 (2001).
Steinhubl, S. R. et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 288, 2411–2420 (2002). The main results of the CREDO study of pretreatment and long term therapy with clopidogrel in patients undergoing percutaneous coronary intervention.
Ferreira, S. H., Ubatuba, F. B. & Vane, J. R. Platelets, acute inflammation and inflammatory mediators. Agents Actions 6, 313–317 (1976).
Libby, P. & Simon, D. I. Inflammation and thrombosis: the clot thickens. Circulation 103, 1718–1720 (2001).
Lindemann, S. et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1β synthesis. J. Cell. Biol. 154, 485–490 (2001).
Ridker, P. M., Cushman, M., Stampfer, M. J., Tracy, R. P. & Hennekens, C. H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 336, 973–979 (1997).
Chew, D. P. et al. Effect of clopidogrel added to aspirin before percutaneous coronary intervention on the risk associated with C-reactive protein. Am. J. Cardiol. 88, 672–674 (2001). The first study to suggest that clopidogrel might have an anti-inflammatory effect.
Bhatt, D. L. & Topol, E. J. Need to test the arterial inflammation hypothesis. Circulation 106, 136–140 (2002). An overview of the role of inflammation in cardiovascular disease.
Neumann, F. J. et al. Induction of cytokine expression in leukocytes by binding of thrombin- stimulated platelets. Circulation 95, 2387–2394 (1997).
Ault, K. A. et al. Platelet activation in patients after an acute coronary syndrome: results from the TIMI-12 trial. Thrombolysis in Myocardial Infarction. J. Am. Coll. Cardiol. 33, 634–639 (1999).
Furman, M. I. et al. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 31, 352–358 (1998).
May, A. E. et al. Reduction of monocyte-platelet interaction and monocyte activation in patients receiving antiplatelet therapy after coronary stent implantation. Eur. Heart. J. 18, 1913–1920 (1997).
Silver, M. J. et al. Adjunctive selectin blockade successfully reduces infarct size beyond thrombolysis in the electrolytic canine coronary artery model. Circulation 92, 492–499 (1995).
Wang, K. et al. Recombinant soluble P-selectin glycoprotein ligand-Ig (rPSGL-Ig) attenuates infarct size and myeloperoxidase activity in a canine model of ischemia-reperfusion. Thromb. Haemost. 88, 149–154 (2002).
Wang, K. et al. Prevention of intimal hyperplasia with recombinant soluble P-selectin glycoprotein ligand-immunoglobulin in the porcine coronary artery balloon injury model. J. Am. Coll. Cardiol. 38, 577–582 (2001).
Davi, G. et al. Increased levels of soluble P-selectin in hypercholesterolemic patients. Circulation 97, 953–957 (1998).
Bhatt, D. L. et al. Complementary, additive benefit of clopidogrel and lipid–lowering therapy in patients with atherosclerosis. J. Amer. Coll. Cardiol 35 (Suppl. A), 326 (2000).
Henn, V. et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391, 591–594 (1998).
Phipps, R. P. Atherosclerosis: the emerging role of inflammation and the CD40-CD40 ligand system. Proc. Natl Acad. Sci. USA 97, 6930–6932 (2000). Concise overview of the roles of CD40 in vascular disease.
Schonbeck, U. et al. CD40 ligation induces tissue factor expression in human vascular smooth muscle cells. Am. J. Pathol. 156, 7–14 (2000).
Slupsky, J. R. et al. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb. Haemost. 80, 1008–1014 (1998).
Aukrust, P. et al. Enhanced levels of soluble and membrane-bound CD40 ligand in patients with unstable angina: possible reflection of T lymphocyte and platelet involvement in the pathogenesis of acute coronary syndromes. Circulation 100, 614–620 (1999).
Schonbeck, U., Varo, N., Libby, P., Buring, J. & Ridker, P. M. Soluble CD40L and cardiovascular risk in women. Circulation 104, 2266–2268 (2001).
Andre, P., Nannizzi-Alaimo, L., Prasad, S. K. & Phillips, D. R. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation 106, 896–899 (2002).
Schonbeck, U. & Libby, P. CD40 signaling and plaque instability. Circ. Res. 89, 1092–1103 (2001).
Horton, D. B., Libby, P. & Schonbeck, U. Ligation of CD40 on vascular smooth muscle cells mediates loss of interstitial collagen via matrix metalloproteinase activity. Ann. NY Acad. Sci. 947, 329–336 (2001).
Urbich, C., Dernbach, E., Aicher, A., Zeiher, A. M. & Dimmeler, S. CD40 ligand inhibits endothelial cell migration by increasing production of endothelial reactive oxygen species. Circulation 106, 981–986 (2002).
Schonbeck, U., Sukhova, G. K., Shimizu, K., Mach, F. & Libby, P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc. Natl Acad. Sci. USA 97, 7458–7463 (2000).
Steinhubl, S. R., Ellis, S. G., Wolski, K., Lincoff, A. M. & Topol, E. J. Ticlopidine pretreatment before coronary stenting is associated with sustained decrease in adverse cardiac events: data from the evaluation of platelet IIb/IIIa inhibitor for stenting (EPISTENT) trial. Circulation 103, 1403–1409 (2001).
Hermann, A., Weber, A. A. & Schror, K. Clopidogrel inhibits platelet adhesion and platelet-dependent mitogenesis in vascular smooth muscle cells. Thromb. Res. 105, 173–175 (2002).
Hermann, A., Rauch, B. H., Braun, M., Schror, K. & Weber, A. A. Platelet CD40 ligand (CD40L)—subcellular localization, regulation of expression, and inhibition by clopidogrel. Platelets 12, 74–82 (2001). A small but important study that demonstrated the ability of clopidogrel to reduce expression of CD40L, a trait not shared by aspirin.
von Hundelshausen, P. et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation 103, 1772–1777 (2001).
Schober, A. et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation 106, 1523–1529 (2002).
Merten, M., Pakala, R., Thiagarajan, P. & Benedict, C. R. Platelet microparticles promote platelet interaction with subendothelial matrix in a glycoprotein IIb/IIIa-dependent mechanism. Circulation 99, 2577–2582 (1999).
Forlow, S. B., McEver, R. P. & Nollert, M. U. Leukocyte-leukocyte interactions mediated by platelet microparticles under flow. Blood 95, 1317–1323 (2000).
Kagawa, H., Nomura, S., Nagahama, M., Ozaki, Y. & Fukuhara, S. Effect of ticlopidine on platelet-derived microparticles in patients with connective tissue diseases. Haemostasis 29, 255–261 (1999).
Storey, R. F., Judge, H. M., Wilcox, R. G. & Heptinstall, S. Inhibition of ADP-induced P-selectin expression and platelet-leukocyte conjugate formation by clopidogrel and the P2Y12 receptor antagonist AR- C69931MX but not aspirin. Thromb. Haemost. 88, 488–494 (2002).
Storey, F. The P2Y12 receptor as a therapeutic target in cardiovascular disease. Platelets 12, 197–209 (2001).
Oury, C. et al. The ATP-gated P2X1 ion channel acts as a positive regulator of platelet responses to collagen. Thromb. Haemost. 86, 1264–1271 (2001).
Offermanns, S., Toombs, C. F., Hu, Y. H. & Simon, M. I. Defective platelet activation in G α(q)-deficient mice. Nature 389, 183–186 (1997).
Ohlmann, P. et al. ADP induces partial platelet aggregation without shape change and potentiates collagen-induced aggregation in the absence of Gαq. Blood 96, 2134–2139 (2000).
Toth-Zsamboki, E., Oury, C., Tytgat, J., Vermylen, J. & Hoylaerts, M. F. The P2Y1 receptor antagonist adenosine-2',5'-diphosphate non- selectively antagonizes the platelet P2X1 ion channel. Thromb. Haemost. 86, 1338–1339 (2001).
Hollopeter, G. et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409, 202–207 (2001). An important description of the particular ADP receptor through which clopidogrel acts.
Fabre, J. E. et al. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nature Med. 5, 1199–202 (1999).
Leon, C. et al. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J. Clin. Invest. 104, 1731–1737 (1999).
Remijn, J. A. et al. Role of ADP receptor P2Y(12) in platelet adhesion and thrombus formation in flowing blood. Arterioscler. Thromb. Vasc. Biol. 22, 686–691 (2002).
Goto, S., Tamura, N., Eto, K., Ikeda, Y. & Handa, S. Functional significance of adenosine 5'-diphosphate receptor (P2Y(12)) in platelet activation initiated by binding of von Willebrand factor to platelet GP Ibα induced by conditions of high shear rate. Circulation 105, 2531–2536 (2002).
Turner, N. A., Moake, J. L. & McIntire, L. V. Blockade of adenosine diphosphate receptors P2Y(12) and P2Y(1) is required to inhibit platelet aggregation in whole blood under flow. Blood 98, 3340–3345 (2001).
Hirsch, E. et al. Resistance to thromboembolism in PI3Kγ-deficient mice. FASEB J 15, 2019–2021 (2001).
Brass, S. Cardiovascular biology. Small cells, big issues. Nature 409, 145–147 (2001).
Enjyoji, K. et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nature Med. 5, 1010–1017 (1999).
Kaneider, N. C. et al. Reversal of thrombin-induced deactivation of CD39/ATPDase in endothelial cells by HMG-CoA reductase inhibition: effects on Rho- GTPase and adenosine nucleotide metabolism. Arterioscler. Thromb. Vasc. Biol. 22, 894–900 (2002).
Pinsky, D. J. et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J. Clin. Invest. 109, 1031–1040 (2002).
Weksler, B. B. Antiplatelet agents in stroke prevention. combination therapy: present and future. Cerebrovasc. Dis. 10, 41–48 (2000).
Anderluh, M. & Dolenc, M. S. Thrombin receptor antagonists; recent advances in PAR-1 antagonist development. Curr. Med. Chem. 9, 1229–1250 (2002).
Brass, S. Cardiovascular biology. Platelets and proteases. Nature 413, 26–27 (2001).
Kim, S. et al. Protease-activated receptors 1 and 4 do not stimulate G(i) signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of G(i) signaling. Blood 99, 3629–3636 (2002).
Sambrano, G. R., Weiss, E. J., Zheng, Y. W., Huang, W. & Coughlin, S. R. Role of thrombin signaling in platelets in haemostasis and thrombosis. Nature 413, 74–78 (2001).
Kahn, M. L., Nakanishi-Matsui, M., Shapiro, M. J., Ishihara, H. & Coughlin, S. R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 103, 879–887 (1999).
Wu, C. C. et al. Selective inhibition of protease-activated receptor 4-dependent platelet activation by YD-3. Thromb. Haemost. 87, 1026–1033 (2002).
Bahou, W. F. Attacked from within, blood thins. Nature Med. 8, 1082–1083 (2002).
Covic, L., Misra, M., Badar, J., Singh, C. & Kuliopulos, A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nature Med. 8, 1161–1165 (2002).
Covic, L., Gresser, A. L., Talavera, J., Swift, S. & Kuliopulos, A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc. Natl Acad. Sci. USA 99, 643–648 (2002).
Bodary, P. F., Westrick, R. J., Wickenheiser, K. J., Shen, Y. & Eitzman, D. T. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA 287, 1706–1709 (2002).
Konstantinides, S., Schafer, K., Koschnick, S. & Loskutoff, D. J. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J. Clin. Invest. 108, 1533–1540 (2001).
Konstantinides, S., Schafer, K. & Loskutoff, D. J. The prothrombotic effects of leptin possible implications for the risk of cardiovascular disease in obesity. Ann. NY Acad. Sci. 947, 134–141; discussion 141–142 (2001).
Nakata, M., Yada, T., Soejima, N. & Maruyama, I. Leptin promotes aggregation of human platelets via the long form of its receptor. Diabetes 48, 426–429 (1999).
Li, Z., Kim, E. S. & Bearer, E. L. Arp2/3 complex is required for actin polymerization during platelet shape change. Blood 99, 4466–4474 (2002).
Barnes, C. S. et al. Production and characterization of saratin, an inhibitor of von Willebrand factor-dependent platelet adhesion to collagen. Semin. Thromb. Hemost. 27, 337–348 (2001).
Kageyama, S., Yamamoto, H. & Yoshimoto, R. Anti-human von willebrand factor monoclonal antibody AJvW-2 prevents thrombus deposition and neointima formation after balloon injury in guinea pigs. Arterioscler. Thromb. Vasc. Biol. 20, 2303–2308 (2000).
Cauwenberghs, N. et al. Antithrombotic effect of platelet glycoprotein Ib-blocking monoclonal antibody Fab fragments in nonhuman primates. Arterioscler. Thromb. Vasc. Biol. 20, 1347–1353 (2000).
Massberg, S. et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 196, 887–896 (2002).
Angelillo-Scherrer, A. et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nature Med. 7, 215–221 (2001).
Topol, E. J. et al. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation 104, 2641–2644 (2001). Report demonstrating the value of SNPs in predicting risk for cardiovascular disease.
Bonnefoy, A., Hantgan, R., Legrand, C. & Frojmovic, M. M. A model of platelet aggregation involving multiple interactions of thrombospondin-1, fibrinogen, and GPIIbIIIa receptor. J. Biol. Chem. 276, 5605–5612 (2001).
Ramakrishnan, V. et al. A thrombin receptor function for platelet glycoprotein Ib-IX unmasked by cleavage of glycoprotein V. Proc. Natl Acad. Sci. USA 98, 1823–1828 (2001).
Kunicki, T. J. The influence of platelet collagen receptor polymorphisms in hemostasis and thrombotic disease. Arterioscler. Thromb. Vasc. Biol. 22, 14–20 (2002).
Murata, M. et al. Coronary artery disease and polymorphisms in a receptor mediating shear stress-dependent platelet activation. Circulation 96, 3281–3286 (1997).
Mikkelsson, J., Perola, M., Penttila, A. & Karhunen, P. J. Platelet glycoprotein Ibα HPA-2 Met/VNTR B haplotype as a genetic predictor of myocardial infarction and sudden cardiac death. Circulation 104, 876–880 (2001).
Huizinga, E. G. et al. Structures of glycoprotein Ibα and its complex with von Willebrand factor A1 domain. Science 297, 1176–1179 (2002).
Sadler, J. E. Biomedicine. Contact—how platelets touch von Willebrand factor. Science 297, 1128–1129 (2002).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
FURTHER INFORMATION
Encyclcopedia of Life Sciences
Glossary
- MYOCARDIAL INFARCTION
-
Popularly known as a heart attack, this is the death of part of the heart muscle due to sudden loss of blood supply. Typically, the loss of this supply is caused by a complete blockage of a coronary artery by a blood clot.
- CAROTID STENOSIS
-
Plaque build-up within the major arteries to the brain, which can predispose to stroke.
- GLANZMANN'S THROMBASTHENIA
-
An autosomal recessive bleeding disorder due to defects in the glycoprotein IIb/IIIa receptor.
- APOPTOSIS
-
Programmed cell death, which is mediated in large part by a family of intracellular cysteine proteases known as caspases.
- CLAUDICATION
-
Pain that occurs with walking, due to atherosclerosis of the arteries in the legs.
- NEUTROPENIA
-
An abnormally low white blood cell count, predisposing to the development of serious infections.
- THROMBOTIC THROMBOCYTOPENIC PURPURA
-
A rare disorder characterized by low platelet counts associated with thrombosis.
- CORONARY STENTING
-
A common minimally invasive procedure in which a metallic stent is placed in the coronary circulation to relieve an obstruction due to atherosclerosis.
- CAROTID STENTING
-
A newer technique to treat carotid stenosis in a minimally invasive manner.
- SURGICAL CAROTID ENDARTERECTOMY
-
The historical way in which to treat carotid stenosis using a conventional surgical incision in the neck.
- ADIPOCYTES
-
The type of cells that make up fat.
Rights and permissions
About this article
Cite this article
Bhatt, D., Topol, E. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2, 15–28 (2003). https://doi.org/10.1038/nrd985
Issue Date:
DOI: https://doi.org/10.1038/nrd985
This article is cited by
-
Therapeutic management of ischemic stroke
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Massive image-based single-cell profiling reveals high levels of circulating platelet aggregates in patients with COVID-19
Nature Communications (2021)
-
Antiplatelet and antithrombotic effects of cordycepin-enriched WIB-801CE from Cordyceps militaris ex vivo, in vivo, and in vitro
BMC Complementary and Alternative Medicine (2016)
-
Platelet function and ageing
Mammalian Genome (2016)
-
Onion (Allium cepa L.) peel extract has anti-platelet effects in rat platelets
SpringerPlus (2015)