Key Points
-
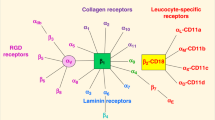
Members of the integrin family of adhesion molecules are non-covalently-associated α/β heterodimers that mediate cell–cell, cell–extracellular matrix and cell–pathogen interactions by binding to distinct, but often overlapping, combinations of ligands.
-
Dysregulation of integrins is involved in the pathogenesis of many disease states, from autoimmunity and thrombotic vascular diseases to cancer metastasis, and so extensive efforts have been directed towards the discovery and development of integrin antagonists for clinical applications. Integrin antagonists are already well established as therapeutics for cardiovascular disease, and applications in other therapuetic areas, including inflammatory disease, seem extremely promising.
-
Integrin ligand-binding function is tightly linked to molecular conformation. On activation, dramatic rearrangements occur in the overall spatial relationships of integrin domains. Understanding the structural basis of integrin activation in detail is essential for understanding the mechanism of antagonism by therapeutics, as well as for the design of second-generation antagonists with novel mechanisms of action.
-
This review discusses examples of the three different classes of integrin antagonists discovered so far: α/β I-like competitive antagonists, α/β I-like allosteric antagonists and α I allosteric antagonists. These examples were chosen because they illustrate particularly well the mutually beneficial relationship between integrin drug discovery and our understanding of integrin structure and function.
Abstract
Integrins are a structurally elaborate family of adhesion molecules that transmit signals bi-directionally across the plasma membrane by undergoing large-scale structural rearrangements. By regulating cell–cell and cell–matrix contacts, integrins participate in a wide range of biological processes, including development, tissue repair, angiogenesis, inflammation and haemostasis. From a therapeutic standpoint, integrins are probably the most important class of cell-adhesion receptors. Recent progress in the development of integrin antagonists has resulted in their clinical application and has shed new light on integrin biology. On the basis of their mechanism of action, small-molecule integrin antagonists fall into three different classes. Each of these classes affect the equilibria that relate integrin conformational states, but in different ways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Hynes, R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25 (1992).
Springer, T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multi-step paradigm. Cell 76, 301–314 (1994).
Humphries, M. J. Integrin structure. Biochem. Soc. Trans. 28, 311–339 (2000).
Shimaoka, M., Takagi, J. & Springer, T. A. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31, 485–516 (2002). Comprehensively reviews conformational changes of integrins with emphasis on the conformational regulation of ligand binding by I domains.
Curley, G. P., Blum, H. & Humphries, M. J. Integrin antagonists. Cell. Mol. Life Sci. 56, 427–441 (1999).
Scarborough, R. M. & Gretler, D. D. Platelet glycoprotein IIb-IIIa antagonists as prototypical integrin blockers: novel parenteral and potential oral antithrombotic agents. J. Med. Chem. 43, 3453–3473 (2000).
Varner, J. A. & Cheresh, D. A. Tumor angiogenesis and the role of vascular cell integrin αvβ3. Important Adv. Oncol. 69–87 (1996).
Giblin, P. A. & Kelly, T. A. Antagonists of β2 integrin-mediated cell adhesion. Annu. Rep. Med. Chem. 36, 181–190 (2001).
Yusuf-Makagiansar, H., Anderson, M. E., Yakovleva, T. V., Murray, J. S. & Siahaan, T. J. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med. Res. Rev. 22, 146–167 (2002).
Bennett, J. S. Novel platelet inhibitors. Annu. Rev. Med. 52, 161–184 (2001).
Cather, J. C. & Menter, A. Modulating T cell responses for the treatment of psoriasis: a focus on efalizumab. Expert Opin. Biol. Ther. 3, 361–370 (2003).
Harlan, J. M. & Winn, R. K. Leukocyte–endothelial interactions: clinical trials of anti-adhesion therapy. Crit. Care Med. 30, S214–S219 (2002).
Harlan, J. M., Winn, R. K., Vedder, N. B., Doerschuk, C. M. & Rice, C. L. in Adhesion: Its Role in Inflammatory Disease (eds Harlan, J. R. & Liu, D.) 117–150 (W. H. Freeman, New York, 1992).
Jackson, D. Y. α4 integrin antagonists. Curr. Pharm. Des. 8, 1229–1253 (2002).
Lin, K. C. & Castro, A. C. Very late antigen 4 (VLA4) antagonists as anti-inflammatory agents. Curr. Opin. Chem. Biol. 2, 453–457 (1998).
Liu, G. Inhibitors of LFA-1/ICAM-1 interaction: from monoclonal antibodies to small molecules. Drugs Future 26, 767–778 (2001).
Tilley, J. W., Chen, L., Sidduri, A. & Fotouhi, N. The discovery of VLA-4 antagonists. Curr. Med. Chem. Rev. (in the press).
Xiong, J. -P. et al. Crystal structure of the extracellular segment of integrin αVβ3. Science 294, 339–345 (2001). The landmark determination of the crystal structure of αvβ3, which demonstrated an unexpected V-shape, or the bent conformation.
Beglova, N., Blacklow, S. C., Takagi, J. & Springer, T. A. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nature Struct. Biol. 9, 282–287 (2002). NMR structure determination of integrin EGF-like domains where activating and activation-dependent epitopes map. Superposition of the integrin EGF-like domains onto the structure of αvβ3 showed these epitopes buried in the bent conformation, strongly indicating that it represents the low-affinity conformation.
Du, X. et al. Long range propagation of conformational changes in integrin αIIbβ3. J. Biol. Chem. 268, 23087–23092 (1993).
Takagi, J., Erickson, H. P. & Springer, T. A. C-terminal opening mimics 'inside-out' activation of integrin α5β1. Nature Struct. Biol. 8, 412–416 (2001).
Weisel, J. W., Nagaswami, C., Vilaire, G. & Bennett, J. S. Examination of the platelet membrane glycoprotein IIb–IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J. Biol. Chem. 267, 16637–16643 (1992).
Takagi, J., Petre, B. M., Walz, T. & Springer, T. A. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–611 (2002). EM image reconstruction, hydrodynamic and ligand-binding studies on αvβ3 revealed that low-affinity bent conformation is converted by activation to the high-affinity extended conformation, which is further stabilized by ligand binding.
Luo, B. -H., Springer, T. A. & Takagi, J. Stabilizing the open conformation of the integrin headpiece with a glycan wedge increases affinity for ligand. Proc. Natl Acad. Sci. USA 100, 2403–2408 (2003).
Lee, J. -O., Rieu, P., Arnaout, M. A. & Liddington, R. Crystal structure of the A domain from the α subunit of integrin CR3 (CD11b/CD18). Cell 80, 631–638 (1995).
Huang, C., Zang, Q., Takagi, J. & Springer, T. A. Structural and functional studies with antibodies to the integrin β2 subunit: a model for the I-like domain. J. Biol. Chem. 275, 21514–21524 (2000).
Springer, T. A. Folding of the N-terminal, ligand-binding region of integrin α-subunits into a β-propeller domain. Proc. Natl Acad. Sci. USA 94, 65–72 (1997).
Diamond, M. S., Garcia-Aguilar, J., Bickford, J. K., Corbi, A. L. & Springer, T. A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J. Cell Biol. 120, 1031–1043 (1993).
Michishita, M., Videm, V. & Arnaout, M. A. A novel divalent cation-binding site in the A domain of the β2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell 72, 857–867 (1993).
Shimaoka, M., Lu, C., Palframan, R., von Andrian, U. H., Takagi, J. & Springer, T. A. Reversibly locking a protein fold in an active conformation with a disulfide bond: integrin αL I domains with high affinity and antagonist activity in vivo. Proc. Natl Acad. Sci. USA 98, 6009–6014 (2001). An engineered disulphide bridge to lock the open conformation of the αL I domain resulted in 10,000-fold increase in ligand-binding affinity to ICAM-1.
Huth, J. R. et al. NMR and mutagenesis evidence for an I domain allosteric site that regulates lymphocyte function-associated antigen 1 ligand binding. Proc. Natl Acad. Sci. USA 97, 5231–5236 (2000).
Shimaoka, M. et al. Structures of the αL I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112, 99–111 (2003). Crystal structure determination of the multiple conformations of the αL I domain with distinct ligand-binding affinity demonstrated shape-shifting pathway for activation by a downward movement of the C-terminal helix.
Emsley, J., Knight, C. G., Farndale, R. W., Barnes, M. J. & Liddington, R. C. Structural basis of collagen recognition by integrin α2β1. Cell 101, 47–56 (2000). The important determination of the open conformation of the α2 I domain in complex with collagen-like peptides demonstrated the significance of the conformational changes induced by ligand binding.
Takagi, J., Kamata, T., Meredith, J., Puzon-McLaughlin, W. & Takada, Y. Changing ligand specificities of αvβ1 and αvβ3 integrins by swapping a short diverse sequence of the β subunit. J. Biol. Chem. 272, 19794–19800 (1997).
Xiong, J. P. et al. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science 296, 151–155 (2002).
Takagi, J. & Springer, T. A. Integrin activation and structural rearrangement. Immunological Rev. 186, 141–163 (2002).
Puzon-McLaughlin, W., Kamata, T. & Takada, Y. Multiple discontinuous ligand-mimetic antibody binding sites define a ligand binding pocket in integrin αIIbβ3. J. Biol. Chem. 275, 7795–7802 (2000).
Kamata, T., Tieu, K. K., Springer, T. A. & Takada, Y. Amino acid residues in the αIIb subunit that are critical for ligand binding to integrin αIIbβ3 are clustered in the β-propeller model. J. Biol. Chem. 276, 44275–44283 (2001).
Luo, B. -H., Springer, T. A. & Takagi, J. High affinity ligand binding by integrins does not involve head separation. J. Biol. Chem. 178, 17185–17189 (2003).
Lu, C., Shimaoka, M., Ferzly, M., Oxvig, C., Takagi, J. & Springer, T. A. An isolated, surface-expressed I domain of the integrin αLβ2 is sufficient for strong adhesive function when locked in the open conformation with a disulfide. Proc. Natl Acad. Sci. USA 98, 2387–2392 (2001).
Lu, C., Shimaoka, M., Zang, Q., Takagi, J. & Springer, T. A. Locking in alternate conformations of the integrin αLβ2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc. Natl Acad. Sci. USA 98, 2393–2398 (2001).
Alonso, J. L., Essafi, M., Xiong, J. P., Stehle, T. & Arnaout, M. A. Does the integrin αA domain act as a ligand for its βA domain? Curr. Biol. 12, R340–R342 (2002).
Salas, A., Shimaoka, M., Chen, S., Carman, C. V. & Springer, T. A. Transition from rolling to firm adhesion is regulated by the conformation of the I domain of the integrin LFA-1. J. Biol. Chem. 277, 50255–50262 (2002).
Dustin, M. L. & Springer, T. A. T cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature 341, 619–624 (1989).
Lollo, B. A., Chan, K. W. H., Hanson, E. M., Moy, V. T. & Brian, A. A. Direct evidence for two affinity states for lymphocyte function-associated antigen 1 on activated T cells. J. Biol. Chem. 268, 21693–21700 (1993).
Constantin, G. et al. Chemokines trigger immediate β2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity 13, 759–769 (2000).
Vinogradova, O. et al. A structural mechanism of integrin αIIbβ3 'inside-out' activation as regulated by its cytoplasmic face. Cell 110, 587–597 (2002). A direct association of the αIIb and β3 cytoplasmic tails was demonstrated by NMR structure determination. The association was perturbed by talin head domain or activating mutations in the tail, supporting integrin activation by separation of the cytoplasmic tails.
Lu, C., Takagi, J. & Springer, T. A. Association of the membrane-proximal regions of the α and β subunit cytoplasmic domains constrains an integrin in the inactive state. J. Biol. Chem. 276, 14642–14648 (2001).
Kim, M., Carman, C. V. & Springer, T. A. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science (in the press). FRET analysis using CFP and YFP fused to the αL and β2 cytoplasmic tails demonstrated separation of the tails in living cells on activation by chemokine and talin head domain as well as on ligand-binding to ICAM-1 in the presence of Mn2+.
Bednar, R. A. et al. Identification of low molecular weight GP IIb/IIIa antagonists that bind preferentially to activated platelets. J. Pharmacol. Exp. Ther. 285, 1317–1326 (1998).
Duggan, M. E. et al. Nonpeptide αVβ3 antagonists. 1. Transformation of a potent, integrin-selective αIIbβ3 antagonist into a potent αVβ3 antagonist. J. Med. Chem. 43, 3736–3745 (2000).
Shih, D. T., Edelman, J. M., Horwitz, A. F., Grunwald, G. B. & Buck, C. A. Structure/function analysis of the integrin β1 subunit by epitope mapping. J. Cell Biol. 122, 1361–1371 (1993).
Bazzoni, G., Shih, D. -T., Buck, C. A. & Hemler, M. A. Monoclonal antibody 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J. Biol. Chem. 270, 25570–25577 (1995).
Takagi, J., Isobe, T., Takada, Y. & Saito, Y. Structural interlock between ligand-binding site and stalk-like region of β1 integrin revealed by a monoclonal antibody recognizing conformation-dependent epitope. J. Biochem. (Tokyo) 121, 914–921 (1997).
Lu, C., Ferzly, M., Takagi, J. & Springer, T. A. Epitope mapping of antibodies to the C-terminal region of the integrin β2 subunit reveals regions that become exposed upon receptor activation. J. Immunol. 166, 5629–5637 (2001).
Du, X. et al. Ligands 'activate' integrin αIIbβ3 (platelet GPIIb-IIIa). Cell 65, 409–416 (1991).
Kouns, W. C. et al. Reversible conformational changes induced in glycoprotein IIb-IIIa by a potent and selective peptidomimetic inhibitor. Blood 80, 2539–2547 (1992).
Honda, S. et al. Association between ligand-induced conformational changes of integrin αIIbβ3 and αIIbβ3-mediated intracellular Ca2+ signaling. Blood 92, 3675–3683 (1998).
Muller, B., Zerwes, H. G., Tangemann, K., Peter, J. & Engel, J. Two-step binding mechanism of fibrinogen to αIIbβ3 integrin reconstituted into planar lipid bilayers. J. Biol. Chem. 268, 6800–6808 (1993).
Huber, W. et al. Determination of kinetic constants for the interaction between the platelet glycoprotein IIb-IIIa and fibrinogen by means of surface plasmon resonance. Eur. J. Biochem. 227, 647–656 (1995).
Bednar, B. et al. Flow cytometric measurement of kinetic and equilibrium binding parameters of arginine-glycine-aspartic acid ligands in binding to glycoprotein IIb/IIIa on platelets. Cytometry 28, 58–65 (1997).
Murphy, N. P., Pratico, D. & Fitzgerald, D. J. Functional relevance of the expression of ligand-induced binding sites in the response to platelet GP IIb/IIIa antagonists in vivo. J. Pharmacol. Exp. Ther. 286, 945–951 (1998).
Thibault, G. Sodium dodecyl sulfate-stable complexes of echistatin and RGD-dependent integrins: a novel approach to study integrins. Mol. Pharmacol. 58, 1137–1145 (2001).
Thibault, G., Tardif, P. & Lapalme, G. Comparative specificity of platelet αIIbβ3 integrin antagonists. J. Pharmacol. Exp. Ther. 296, 690–696 (2000).
Zolotarjova, N. I., Hollis, G. F. & Wynn, R. Unusually stable and long-lived ligand-induced conformations of integrins. J. Biol. Chem. 276, 17063–17068 (2001). References 64 and 65 describe an 'unusually' tight stabilization by α/β I-like competitive antagonists of integrin α- and β-subunit association.
Billheimer, J. T. et al. Evidence that thrombocytopenia observed in humans treated with orally bioavailable glycoprotein IIb/IIIa antagonists is immune mediated. Blood 99, 1–7 (2002).
Peter, K., Schwarz, M., Nordt, T. & Bode, C. Intrinsic activating properties of GP IIb/IIIa blockers. Thromb. Res. 103, S21–S27 (2001).
Frelinger, A. L., Furman, M. I., Krueger, L. A., Barnard, M. R. & Michelson, A. D. Dissociation of glycoprotein IIb/IIIa antagonists from platelets does not result in fibrinogen binding or platelet aggregation. Circulation 104, 1374–1379 (2001).
Schneider, D. J., Taatjes, D. J. & Sobel, B. E. Paradoxical inhibition of fibrinogen binding and potentiation of α-granule release by specific types of inhibitors of glycoprotein IIb-IIIa. Cardiovasc. Res. 45, 437–446 (2000).
Brown, E. J. & Gresham, R. D. in Structure, Function, and Regulation of Molecules Involved in Leukocyte Adhesion Vol. 1 (eds Lipsky, P. E., Rothlein, R., Kishimoto, T. K., Faanes, R. B. & Smith, C. W.) 78–91 (Springer, New York, 1993).
Varner, J. A. & Cheresh, D. A. Integrins and cancer. Curr. Opin. Cell Biol. 8, 724–730 (1996).
Hynes, R. O. A reevaluation of integrins as regulators of angiogenesis. Nature Med. 8, 918–921 (2002).
Engleman, V. W. et al. A peptidomimetic antagonist of the αVβ3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J. Clin. Invest. 99, 2284–2292 (1997).
Bach, A. C. et al. Type II' to type I β-turn swap changes specificity for integrins. J. Am. Chem. Soc. 118, 293–294 (1996).
Bazzoni, G. & Hemler, M. E. Are changes in integrin affinity and conformation overemphasized? Trends Biochem. Sci. 23, 30–34 (1998).
Honda, S. et al. Ligand binding to integrin αvβ3 requires tyrosine 178 in the αv subunit. Blood 97, 175–182 (2001).
Legler, D. F., Wiedle, G., Ross, F. P. & Imhof, B. A. Superactivation of integrin αVβ3 by low antagonist concentrations. J. Cell Sci. 114, 1545–1553 (2001).
Hynes, R. O. Integrins: bi-directional, allosteric, signalling machines. Cell 110, 673–687 (2002). Excellently reviews the latest developments in integrin biology and structure.
Yednock, T. A. et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature 356, 63–66 (1992).
Miller, D. H. et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348, 15–23 (2003).
Ghosh, S. et al. Natalizumab for active Crohn's disease. N. Engl. J. Med. 348, 24–32 (2003).
Butcher, E. C. & Picker, L. J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Wang, J. -H. & Springer, T. A. Structural specializations of immunoglobulin superfamily members for adhesion to integrins and viruses. Immunol. Rev. 163, 197–215 (1998).
Copie, V. et al. Solution structure and dynamics of linked cell attachment modules of mouse fibronectin containing the RGD and synergy regions: comparison with the human fibronectin crystal structure. J. Mol. Biol. 277, 663–682 (1998).
Chen, L. L. et al. Identification of ligand binding sites on integrin α4β1 through chemical cross-linking. Biochemistry 37, 8743–8753 (1998).
Irie, A., Kamata, T. & Takada, Y. Multiple loop structures critical for ligand binding of the integrin α4 subunit in the upper face of the β-propeller models. Proc. Natl Acad. Sci. USA 94, 7198–7203 (1997).
Yednock, T. A. et al. α4β1 integrin-dependent cell adhesion is regulated by a low affinity receptor pool that is conformationally responsive to ligand. J. Biol. Chem. 270, 28740–28750 (1995).
Chigaev, A. et al. Real-time analysis of the affinity regulation of α4-integrin: the physiologically activated receptor is intermediate in affinity between resting and Mn2+ or antibody activation. J. Biol. Chem. 276, 48670–48678 (2001).
Dustin, M. L. & Springer, T. A. in Guidebook to the Extracellular Matrix and Adhesion Proteins (eds Kreis, T. & Vale, R.) 228–232 (Sambrook and Tooze, New York, 1999).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Gottlieb, A. et al. Effects of administration of a single dose of a humanized monoclonal antibody to CD11a on the immunobiology and clinical activity of psoriasis. J. Am. Acad. Dermatol. 42, 428–435 (2000).
Dustin, M. L. & Springer, T. A. in Guidebook to the Extracellular Matrix and Adhesion Proteins (eds Kreis, T. & Vale, R.) 216–220 (Sambrook and Tooze, New York, 1999).
Diamond, M. S., Staunton, D. E., Marlin, S. D. & Springer, T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third Ig-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell 65, 961–971 (1991).
Huang, C. & Springer, T. A. A binding interface on the I domain of lymphocyte function associated antigen-1 (LFA-1) required for specific interaction with intercellular adhesion molecule 1 (ICAM-1). J. Biol. Chem. 270, 19008–19016 (1995).
Kallen, J. et al. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J. Mol. Biol. 292, 1–9 (1999).
Last-Barney, K. et al. Binding site elucidation of hydantoin-based antagonists of LFA-1 using multidisciplinary technologies: evidence for the allosteric inhibition of a protein–protein interaction. J. Am. Chem. Soc. 123, 5643–5650 (2001).
Liu, G. et al. Novel p-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intracellular adhesion molecule-1 interaction. 2. Mechanism of inhibition and structure-based improvement of pharmaceutical properties. J. Med. Chem. 44, 1202–1210 (2001). References 95, 96 and 97 crystallographically demonstrated that a class of small-molecule antagonists to αLβ2 bound beneath the C-terminal α-helix of the αL I domain, providing a structural basis for α I allosteric antagonists.
Welzenbach, K., Hommel, U. & Weitz-Schmidt, G. Small molecule inhibitors induce conformational changes in the I domain and the I-like domain of lymphocyte function-associated antigen-1: molecular insights into integrin inhibition. J. Biol. Chem. 277, 10590–10598 (2002).
Woska, J. R. Jr et al. A small-molecule antagonist of LFA-1 blocks a conformational change important for LFA-1 function. J. Leukoc. Biol. 70, 329–334 (2001).
Shimaoka, M., Salas, A., Yang, W., Weitz-Schmidt, G. & Springer, T. A. Small molecule integrin antagonists that bind to the β2 subunit I-like domain and activate signals in one direction and block them in another. Immunity (in the press). Describes a novel mechanistic class of integrin inhibitors, α/β I-like allosteric antagonists that bind to the MIDAS of the β2 I-like domain and disrupt interdomain communication between the I and I-like domains. While blocking conformational signal transmission to the I domain, the antagonists activate the I-like domain by mimicking an internal ligand, a conserved acidic residue in the I domain linker.
Fotouhi, N., Gillespie, P., Guthrie, R., Pietranico-Cole, S. & Yun, W. Diaminopropionic acid derivatives. PCT Int. Appl. Hoffmann–La Roche, Switzerland, WO0021920 (1999).
Burdick, D. J. Antagonists for treatment of CD11/CD18 adhesion receptor mediated disorders. PCT Int. Appl. Genentech, USA, WO9949856 (1999).
Gadek, T. R. et al. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science 295, 1086–1089 (2002).
Hesterberg, P. E. et al. Rapid resolution of chronic colitis with an antibody to a gut homing integrin α4β7. Gastroenterology 111, 1373–1380 (1996).
de Fougerolles, A. R. et al. Regulation of inflammation by collagen-binding integrins α1β1 and α2β1 in models of hypersensitivity and arthritis. J. Clin. Invest. 105, 721–729 (2000).
Doolittle, R. F. Fibrinogen and fibrin. Sci. Am. 245, 126–135 (1981).
Henschen, A., Lottspeich, F., Kehl, M. & Southan, C. Covalent structure of fibrinogen. Ann. NY Acad. Sci. 408, 28–43 (1983).
Springer, T. A. Predicted and experimental structures of integrins and β-propellers. Curr. Opin. Struct. Biol. 12, 802–813 (2002).
Coleman, P. J. et al. Non-peptide αVβ3 antagonists. Part 3: identification of potent RGD mimetics incorporating novel β-amino acids as aspartic acid replacements. Bioorg. Med. Chem. Lett. 12, 31–34 (2002).
Ward, K. W. et al. Preclinical pharmacokinetics and interspecies scaling of a novel vitronectin receptor antagonist. Drug Metab. Dispos. 27, 1232–1241 (1999).
Weitz-Schmidt, G. et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature Med. 7, 687–692 (2001).
Acknowledgements
We would like to thank T. Xiao and W. Yang for modelling of the αIIbβ3 headpiece, and J. Takagi, T. Vorup-Jensen, B.-H. Luo, M. Kim, G. Weitz-Schmidt, T. A. Kelly and J. W. Tilley for critically reading this manuscript.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
LocusLink
Online Mendelian Inheritance in Man
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- INTEGRIN-EGF DOMAIN
-
A module in cysteine-rich repeats in the integrin β-subunit stalk region adopts a nosecone-shaped variant of the epidermal growth factor (EGF) fold, termed an integrin-EGF (I-EGF) domain.
- SDS–PAGE
-
(Sodium dodecyl sulphate– polyacrylamide gel electrophoresis). A method for resolving a protein into its subunits and determining their separate molecular weights.
- THROMBOCYTOPAENIA
-
A disorder in which the number of platelets is abnormally low, and which is sometimes associated with abnormal bleeding.
- IMMUNOLOGICAL SYNAPSE
-
T-cell recognition of an antigen presenting cell (APC), which is the initial and crucial process in the antigen-specific immune response, takes place at the nanometer-scale gap of the interface between the T cell and APC. This interface is a specialized cell–cell junction, at which crucial signals to initiate and maintain the immune response are transduced from APC to T cell or vice versa. The interface is called an immunological synapse after the neuronal synapse, a segregated gap through which information is transmitted in chemical form (neurotransmitter) from one neuron to another.
Rights and permissions
About this article
Cite this article
Shimaoka, M., Springer, T. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov 2, 703–716 (2003). https://doi.org/10.1038/nrd1174
Issue Date:
DOI: https://doi.org/10.1038/nrd1174
This article is cited by
-
Modelling the complex nature of the tumor microenvironment: 3D tumor spheroids as an evolving tool
Journal of Biomedical Science (2024)
-
Anoikis resistance––protagonists of breast cancer cells survive and metastasize after ECM detachment
Cell Communication and Signaling (2023)
-
Lymphocyte integrins mediate entry and dysregulation of T cells by SARS-CoV-2
Signal Transduction and Targeted Therapy (2023)
-
Structural analysis of peptide binding to integrins for cancer detection and treatment
Biophysical Reviews (2023)
-
Structural basis for adhesion G protein-coupled receptor Gpr126 function
Nature Communications (2020)