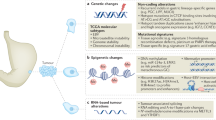
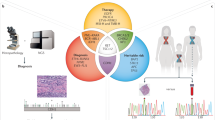
Abstract
Cancer is a leading cause of death worldwide. Great efforts are dedicated to the development of prognostic and predictive biomarkers to improve diagnosis and achieve optimal treatment selection, thereby, introducing precision medicine in the multimodality treatment of cancer. Genomic aberrations are the basis of tumour development, representing excellent candidates for the development of promising clinical biomarkers. Over the past decade, single-gene mutations and genomic profiling have been increasingly used in multidisciplinary consultations for risk-assessment and treatment planning for patients with cancer. We discuss the impact of such genetic-based information on surgical decision-making. Single-gene mutations have already influenced surgical decision-making in breast, colorectal and thyroid cancer. However, the direct impact of genomic profiling on surgical care has not yet been fully established. We discuss the direct and indirect influences of genomic profiling on surgery, and analyse the limitations and unresolved issues of a genotypic-approach to the surgical management of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 (2012).
Amado, R. G. et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634 (2008).
Armaghany, T., Wilson, J. D., Chu, Q. & Mills, G. Genetic alterations in colorectal cancer. Gastrointest. Cancer Res. 5, 19–27 (2012).
Giardiello, F. M. et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N. Engl. J. Med. 328, 1313–1316 (1993).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Labayle, D. et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 101, 635–639 (1991).
Ladenheim, J. et al. Effect of sulindac on sporadic colonic polyps. Gastroenterology 108, 1083–1087 (1995).
Balmana, J., Diez, O., Rubio, I. & Castiglione, M. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 21 (Suppl. 5), v20–v22 (2010).
Albain, K. S. et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 11, 55–65 (2010).
Gray, R. G. et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J. Clin. Oncol. 29, 4611–4619 (2011).
van 't Veer, L. J. et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 (2002).
Bild, A. H. et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439, 353–357 (2006).
Bride, M. B. et al. Factors associated with surgical decision making in women with early-stage breast cancer: a literature review. J. Womens Health (Larchmt) 22, 236–242 (2013).
Jatoi, I. Options in breast cancer local therapy: who gets what? World J. Surg. 36, 1498–1502 (2012).
Parry, S. et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 60, 950–957 (2011).
Romei, C., Pardi, E., Cetani, F. & Elisei, R. Genetic and clinical features of multiple endocrine neoplasia types 1 and 2. J. Oncol. 2012, 705036 (2012).
Duncan, J. A., Reeves, J. R. & Cooke, T. G. BRCA1 and BRCA2 proteins: roles in health and disease. Mol. Pathol. 51, 237–247 (1998).
Lord, C. J. & Ashworth, A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat. Med. 19, 1381–1388 (2013).
Metcalfe, K. et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br. J. Cancer 104, 1384–1392 (2011).
Miki, Y. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71 (1994).
Robson, M. et al. Appropriateness of breast-conserving treatment of breast carcinoma in women with germline mutations in BRCA1 or BRCA2: a clinic-based series. Cancer 103, 44–51 (2005).
Neuburger, J., Macneill, F., Jeevan, R., van der Meulen, J. H. & Cromwell, D. A. Trends in the use of bilateral mastectomy in England from 2002 to 2011: retrospective analysis of hospital episode statistics. BMJ Open 3, (2013).
Rebbeck, T. R. et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J. Clin. Oncol. 22, 1055–1062 (2004).
Metcalfe, K. A. et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int. J. Cancer 122, 2017–2022 (2008).
Finch, A. et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. JAMA 296, 185–192 (2006).
Hermsen, B. B. et al. No efficacy of annual gynaecological screening in BRCA1/2 mutation carriers; an observational follow-up study. Br. J. Cancer 96, 1335–1342 (2007).
Saha, D., Roman, C. & Beauchamp, R. D. New strategies for colorectal cancer prevention and treatment. World J. Surg. 26, 762–766 (2002).
Warrier, S. K. & Kalady, M. F. Familial adenomatous polyposis: challenges and pitfalls of surgical treatment. Clin. Colon Rectal. Surg. 25, 83–89 (2012).
Schwarzova, L. et al. Novel mutations of the APC gene and genetic consequences of splicing mutations in the Czech FAP families. Fam. Cancer 12, 35–42 (2013).
Church, J., Burke, C., McGannon, E., Pastean, O. & Clark, B. Predicting polyposis severity by proctoscopy: how reliable is it? Dis. Colon Rectum 44, 1249–1254 (2001).
Bulow, S. et al. Colectomy and ileorectal anastomosis is still an option for selected patients with familial adenomatous polyposis. Dis. Colon Rectum 51, 1318–1323 (2008).
Gunther, K., Braunrieder, G., Bittorf, B. R., Hohenberger, W. & Matzel, K. E. Patients with familial adenomatous polyposis experience better bowel function and quality of life after ileorectal anastomosis than after ileoanal pouch. Colorectal Dis. 5, 38–44 (2003).
Olsen, K. O. et al. Female fecundity before and after operation for familial adenomatous polyposis. Br. J. Surg. 90, 227–231 (2003).
Slors, F. J., van Zuijlen, P. P. & van Dijk, G. J. Sexual and bladder dysfunction after total mesorectal excision for benign diseases. Scand. J. Gastroenterol. Suppl. 232, 48–51 (2000).
Markowitz, S. D. & Bertagnolli, M. M. Molecular origins of cancer: Molecular basis of colorectal cancer. N. Engl. J. Med. 361, 2449–2460 (2009).
Martin-Lopez, J. V. & Fishel, R. The mechanism of mismatch repair and the functional analysis of mismatch repair defects in Lynch syndrome. Fam. Cancer 12, 159–168 (2013).
Kohlmann, W. & Gruber, S. B. Lynch Syndrome in Gene Reviews (eds Pagon, R. A. et al.) 1–2 (University of Washington, Seattle, 2004).
Engel, C. et al. Risks of less common cancers in proven mutation carriers with Lynch syndrome. J. Clin. Oncol. 30, 4409–4415 (2012).
Kloos, R. T. et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19, 565–612 (2009).
Kurzrock, R. et al. Tumor marker and measurement fluctuations may not reflect treatment efficacy in patients with medullary thyroid carcinoma on long-term RET inhibitor therapy. Ann. Oncol. 24, 2256–2261 (2013).
Rivkees, S. A. et al. The treatment of differentiated thyroid cancer in children: emphasis on surgical approach and radioactive iodine therapy. Endocr. Rev. 32, 798–826 (2011).
Elisei, R. et al. The timing of total thyroidectomy in RET gene mutation carriers could be personalized and safely planned on the basis of serum calcitonin: 18 years experience at one single center. J. Clin. Endocrinol. Metab. 97, 426–435 (2012).
Xing, M., Haugen, B. R. & Schlumberger, M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 381, 1058–1069 (2013).
Beer, D. G. et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat. Med. 8, 816–824 (2002).
Paik, S. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351, 2817–2826 (2004).
Paik, S. et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 24, 3726–3734 (2006).
Dowsett, M. et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J. Clin. Oncol. 28, 1829–1834 (2010).
Mamounas, E. P. et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J. Clin. Oncol. 28, 1677–1683 (2010).
Glas, A. M. et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics 7, 278 (2006).
van de Vijver, M. J. et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002).
Arpino, G. et al. Gene expression profiling in breast cancer: a clinical perspective. Breast 22, 109–120 (2013).
Cardoso, F. et al. Clinical application of the 70-gene profile: the MINDACT trial. J. Clin. Oncol. 26, 729–735 (2008).
O'Connell, M. J. et al. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J. Clin. Oncol. 28, 3937–3944 (2010).
Salazar, R. et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J. Clin. Oncol. 29, 17–24 (2011).
Maak, M. et al. Independent validation of a prognostic genomic signature (ColoPrint) for patients with stage II colon cancer. Ann. Surg. 257, 1053–1058 (2013).
Ayers, M. et al. Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. J. Clin. Oncol. 22, 2284–2293 (2004).
Chang, J. C. et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet 362, 362–369 (2003).
Cho, J. H. et al. Oncologic safety of breast-conserving surgery compared to mastectomy in patients receiving neoadjuvant chemotherapy for locally advanced breast cancer. J. Surg. Oncol. 108, 531–536 (2013).
Shin, H. C. et al. Breast-conserving surgery after tumor downstaging by neoadjuvant chemotherapy is oncologically safe for stage III breast cancer patients. Ann. Surg. Oncol. 20, 2582–2589 (2013).
Rose, J. B. et al. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann. Surg. Oncol. 21, 1530–1537 (2014).
Hannemann, J. et al. Changes in gene expression associated with response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 23, 3331–3342 (2005).
Hartley, A., Ho, K. F., McConkey, C. & Geh, J. I. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br. J. Radiol. 78, 934–938 (2005).
Habr-Gama, A. et al. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis. Colon Rectum 41, 1087–1096 (1998).
Habr-Gama, A. et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann. Surg. 240, 711–717 (2004).
Habr-Gama, A. et al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J. Gastrointest. Surg. 9, 90–99 (2005).
Habr-Gama, A. et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J. Gastrointest. Surg. 10, 1319–1328 (2006).
Habr-Gama, A. Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Colorectal Dis. 8 (Suppl. 3), 21–24 (2006).
Maas, M. et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J. Clin. Oncol. 29, 4633–4640 (2011).
Smith, F. M., Wiland, H., Mace, A., Pai, R. K. & Kalady, M. F. Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis. Colon Rectum 57, 311–315 (2014).
van Hagen, P. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 366, 2074–2084 (2012).
Kim, J. Y. & Hofstetter, W. L. Esophagectomy after chemoradiation: who and when to operate. Semin. Thorac. Cardiovasc. Surg. 24, 288–293 (2012).
Stahl, M. et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J. Clin. Oncol. 23, 2310–2317 (2005).
Furlong, H. et al. Targeting therapy for esophageal cancer in patients aged 70 and over. J. Geriatr. Oncol. 4, 107–113 (2013).
Edgren, G., Adami, H. O., Weiderpass, E. & Nyren, O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 62, 1406–1414 (2013).
Hong, M. K. et al. Percutaneous image-guided biopsy of prostate cancer metastases yields samples suitable for genomics and personalised oncology. Clin. Exp. Metastasis 31, 159–167 (2013).
Marshall, D., Laberge, J. M., Firetag, B., Miller, T. & Kerlan, R. K. The changing face of percutaneous image-guided biopsy: molecular profiling and genomic analysis in current practice. J. Vasc. Interv. Radiol. 24, 1094–1103 (2013).
Al-Leswas, D., O'Reilly, D. A. & Poston, G. J. Biopsy of solid liver tumors: adverse consequences. Hepatobiliary Pancreat. Dis. Int. 7, 325–327 (2008).
Boutin, C., Rey, F. & Viallat, J. R. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 108, 754–758 (1995).
Jones, O. M., Rees, M., John, T. G., Bygrave, S. & Plant, G. Biopsy of resectable colorectal liver metastases causes tumour dissemination and adversely affects survival after liver resection. Br. J. Surg. 92, 1165–1168 (2005).
Liebens, F. et al. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas 62, 113–123 (2009).
Mesker, W. E. et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 29, 387–398 (2007).
Varga, Z. et al. Comparison of EndoPredict and Oncotype DX test results in hormone receptor positive invasive breast cancer. PLoS ONE 8, e58483 (2013).
McShane, L. M. et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat. Clin. Pract. Oncol. 2, 416–422 (2005).
Glynne-Jones, R. & Hughes, R. Critical appraisal of the 'wait and see' approach in rectal cancer for clinical complete responders after chemoradiation. Br. J. Surg. 99, 897–909 (2012).
de Campos-Lobato, L. F. et al. Neoadjuvant therapy for rectal cancer: the impact of longer interval between chemoradiation and surgery. J. Gastrointest. Surg. 15, 444–450 (2011).
Tulchinsky, H., Shmueli, E., Figer, A., Klausner, J. M. & Rabau, M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann. Surg. Oncol. 15, 2661–2667 (2008).
Ruol, A. et al. Interval between neoadjuvant chemoradiotherapy and surgery for squamous cell carcinoma of the thoracic esophagus: does delayed surgery have an impact on outcome? Ann. Surg. 252, 788–796 (2010).
Buchholz, T. A. et al. Global gene expression changes during neoadjuvant chemotherapy for human breast cancer. Cancer J. 8, 461–468 (2002).
Zujewski, J. A. & Kamin, L. Trial assessing individualized options for treatment for breast cancer: the TAILORx trial. Future Oncol. 4, 603–610 (2008).
Bernards, R. A missing link in genotype-directed cancer therapy. Cell 151, 465–468 (2012).
Ferlay, J. et al. Globocan 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. Globocan 2012 [online], (2012).
Baylin, S. B. & Jones, P. A. A decade of exploring the cancer epigenome - biological and translational implications. Nat. Rev. Cancer 11, 726–734 (2011).
Goel, A. & Boland, C. R. Epigenetics of colorectal cancer. Gastroenterology 143, 1442–1460 (2012).
Sharma, S., Kelly, T. K. & Jones, P. A. Epigenetics in cancer. Carcinogenesis 31, 27–36 (2010).
Arnold, C. N., Goel, A. & Boland, C. R. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int. J. Cancer 106, 66–73 (2003).
Baylin, S. B. Resistance, epigenetics and the cancer ecosystem. Nat. Med. 17, 288–289 (2011).
Huynh, K. T., Chong, K. K., Greenberg, E. S. & Hoon, D. S. Epigenetics of estrogen receptor-negative primary breast cancer. Expert Rev. Mol. Diagn. 12, 371–382 (2012).
Lyko, F. & Brown, R. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J. Natl Cancer Inst. 97, 1498–1506 (2005).
Sekeres, M. A. et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood 120, 4945–4951 (2012).
Garcia-Manero, G. Demethylating agents in myeloid malignancies. Curr. Opin. Oncol. 20, 705–710 (2008).
Huang, Y., Nayak, S., Jankowitz, R., Davidson, N. E. & Oesterreich, S. Epigenetics in breast cancer: what's new? Breast Cancer Res. 13, 225 (2011).
Grady, W. M., Rajput, A., Lutterbaugh, J. D. & Markowitz, S. D. Detection of aberrantly methylated hMLH1 promoter DNA in the serum of patients with microsatellite unstable colon cancer. Cancer Res. 61, 900–902 (2001).
Hellebrekers, D. M. et al. GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin. Cancer Res. 15, 3990–3997 (2009).
Nagasaka, T. et al. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J. Natl Cancer Inst. 101, 1244–1258 (2009).
Ouyang, D. L., Chen, J. J., Getzenberg, R. H. & Schoen, R. E. Noninvasive testing for colorectal cancer: a review. Am. J. Gastroenterol. 100, 1393–1403 (2005).
Heyn, H. & Esteller, M. DNA methylation profiling in the clinic: applications and challenges. Nat. Rev. Genet. 13, 679–692 (2012).
Goessl, C. et al. Fluorescent methylation-specific polymerase chain reaction for DNA-based detection of prostate cancer in bodily fluids. Cancer Res. 60, 5941–5945 (2000).
Sunami, E. et al. Multimarker circulating DNA assay for assessing blood of prostate cancer patients. Clin. Chem. 55, 559–567 (2009).
Yegnasubramanian, S. et al. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 64, 1975–1986 (2004).
Nakayama, H. et al. Molecular detection of p16 promoter methylation in the serum of recurrent colorectal cancer patients. Int. J. Cancer 105, 491–493 (2003).
Yamaguchi, S., Asao, T., Nakamura, J., Ide, M. & Kuwano, H. High frequency of DAP-kinase gene promoter methylation in colorectal cancer specimens and its identification in serum. Cancer Lett. 194, 99–105 (2003).
Tan, S. H. et al. Detection of promoter hypermethylation in serum samples of cancer patients by methylation-specific polymerase chain reaction for tumour suppressor genes including RUNX3. Oncol. Rep. 18, 1225–1230 (2007).
Ebert, M. P. et al. Aristaless-like homeobox-4 gene methylation is a potential marker for colorectal adenocarcinomas. Gastroenterology 131, 1418–1430 (2006).
Lofton-Day, C. et al. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin. Chem. 54, 414–423 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Reimers, M., Engels, C., Kuppen, P. et al. How does genome sequencing impact surgery?. Nat Rev Clin Oncol 11, 610–618 (2014). https://doi.org/10.1038/nrclinonc.2014.101
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.101
This article is cited by
-
WT1 expression in vessels varies with histopathological grade in tumour-bearing and control tissue from patients with breast cancer
British Journal of Cancer (2018)
-
Systems biology approaches to adverse drug effects: the example of cardio-oncology
Nature Reviews Clinical Oncology (2015)