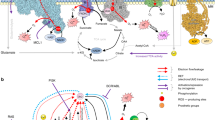
Key Points
-
As early as 1981, it was suggested that enzymes biochemically similar to the NADPH oxidase (NOX) NOX2 were present in all cell types, not just in neutrophils. A decade later, high levels of superoxide and hydrogen peroxide were found in various cancer cells, and these levels could be reduced by diphenyleneiodonium (DPI), a chemical inhibitor of flavoprotein-containing enzymes, such as NOX oxidases.
-
The mitochondrial electron transfer chain and NADPH oxidases of the NOX family are the two major sources of reactive oxygen species (ROS) that are implicated in cancer.
-
Seven membrane-bound NOX catalytic isoforms (NOX1–5) and dual oxidase 1 (DUOX1) and DUOX2 have been identified, each of which displays similar but distinct structural, biochemical and subcellular localization characteristics.
-
NOX-dependent redox signalling occurs at cellular membranes and intracellular structures where the NOX catalytic and regulatory subunits are localized. The NOX catalytic subunits NOX2, NOX1, NOX4 and NOX5 have been detected in the plasma membrane. NOX4 has additionally been detected in the endoplasmic reticulum, mitochondrial and nuclear membranes. NOX subunits also reside at specific subcellular microdomains, such as NOX4 at focal adhesions, NOX1 at caveoli and lipid rafts, and NOX1 and NOX4 at invadopodia.
-
NOX catalytic and regulatory subunits have been implicated in one or more of the most common cancer types. Increased mRNA and/or protein expression of NOX1, NOX2, NOX4 and NOX5 or their regulatory components have been detected at higher levels in various cultured cancer cell lines or human tumours compared with normal controls at early and late stages of tumorigenesis, suggesting that ROS derived from NADPH oxidases may be important in both the initiation and the maintenance phases of tumour development.
-
Initial studies indicate that NOX oxidases can effect several of the hallmarks of cancer, including genomic instability, autonomous growth and survival, angiogenesis, invasion and metastasis.
Abstract
NADPH oxidases of the NADPH oxidase (NOX) family are dedicated reactive oxygen species-generating enzymes that broadly and specifically regulate redox-sensitive signalling pathways that are involved in cancer development and progression. They act at specific cellular membranes and microdomains through the activation of oncogenes and the inactivation of tumour suppressor proteins. In this Review, we discuss primary targets and redox-linked signalling systems that are influenced by NOX-derived ROS, and the biological role of NOX oxidases in the aetiology of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Knudson, A. G. Two genetic hits (more or less) to cancer. Nature Rev. Cancer 1, 157–162 (2001).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Oberley, L. W., Oberley, T. D. & Buettner, G. R. Cell division in normal and transformed cells: the possible role of superoxide and hydrogen peroxide. Med. Hypotheses 7, 21–42 (1981). The free radical theory of cancer is presented.
McCord, J. M. & Fridovich, I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol. Chem. 244, 6056–6063 (1969). This seminal paper describes the biochemical characterizaiton of superoxide dismutases involved in neutralizing ROS.
Dewald, B., Baggiolini, M., Curnutte, J. T. & Babior, B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J. Clin. Invest. 63, 21–29 (1979).
Bittinger, F. et al. Production of superoxide by human malignant melanoma cells. Melanoma Res. 8, 381–387 (1998).
Szatrowski, T. P. & Nathan, C. F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 51, 794–798 (1991). This paper describes the detection of diphenyleneiodonium-inhibitable ROS in a large variety of cancer cells.
Ralph, S. J., Rodriguez-Enriquez, S., Neuzil, J., Saavedra, E. & Moreno-Sanchez, R. The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation - why mitochondria are targets for cancer therapy. Mol. Aspects Med. 31, 145–170 (2010).
Singh, K. K. Mitochondria damage checkpoint, aging, and cancer. Ann. NY Acad. Sci. 1067, 182–190 (2006).
Wallace, D. C. Mitochondria and cancer: Warburg addressed. Cold Spring Harb. Symp. Quant. Biol. 70, 363–374 (2005).
Barnes, J. L. & Gorin, Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 79, 944–956 (2011).
Bedard, K. & Krause, K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 (2007). This is an extensive review of the structure and function of NOX oxidases.
Drummond, G. R., Selemidis, S., Griendling, K. K. & Sobey, C. G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nature Rev. Drug Discov. 10, 453–471 (2011).
Banfi, B. et al. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 279, 46065–46072 (2004).
Banfi, B. et al. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science 287, 138–142 (2000).
Cheng, G., Cao, Z., Xu, X., van Meir, E. G. & Lambeth, J. D. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269, 131–140 (2001).
Dupuy, C. et al. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J. Biol. Chem. 274, 37265–37269 (1999).
Geiszt, M. NADPH oxidases: new kids on the block. Cardiovasc. Res. 71, 289–299 (2006).
Geiszt, M., Kopp, J. B., Varnai, P. & Leto, T. L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl Acad. Sci. USA 97, 8010–8014 (2000). This paper describes the cloning of the NOX isoform NOX4 and suggests that NOX4 is an oxygen sensor.
Lambeth, J. D., Kawahara, T. & Diebold, B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 43, 319–331 (2007).
Shiose, A. et al. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 276, 1417–1423 (2001).
Suh, Y. A. et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401, 79–82 (1999). This paper describes the cloning of the NOX isoform NOX1 and provides the first evidence that a NOX oxidase may mediate cellular transformation.
Ginabreda, M. G. et al. Negative correlation between thyroperoxidase and dual oxidase H2O2-generating activities in thyroid nodular lesions. Eur. J. Endocrinol. 158, 223–227 (2008).
Lacroix, L. et al. Expression of nicotinamide adenine dinucleotide phosphate oxidase flavoprotein DUOX genes and proteins in human papillary and follicular thyroid carcinomas. Thyroid 11, 1017–1023 (2001).
Luxen, S., Belinsky, S. A. & Knaus, U. G. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 68, 1037–1045 (2008).
Block, K., Gorin, Y. & Abboud, H. E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl Acad. Sci. USA 106, 14385–14390 (2009).
Borregaard, N., Heiple, J. M., Simons, E. R. & Clark, R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J. Cell Biol. 97, 52–61 (1983).
Serrander, L. et al. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89, 1159–1167 (2007).
Takac, I. et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 286, 13304–13313 (2011).
von Lohneysen, K., Noack, D., Jesaitis, A. J., Dinauer, M. C. & Knaus, U. G. Mutational analysis reveals distinct features of the Nox4-p22 phox complex. J. Biol. Chem. 283, 35273–35282 (2008).
Van Buul, J. D., Fernandez-Borja, M., Anthony, E. C. & Hordijk, P. L. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid. Redox Signal. 7, 308–317 (2005).
Ago, T. et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 106, 1253–1264 (2010).
Graham, K. A. et al. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol. Ther. 10, 223–231 (2010).
Hilenski, L. L., Clempus, R. E., Quinn, M. T., Lambeth, J. D. & Griendling, K. K. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 24, 677–683 (2004).
Cheng, G. & Lambeth, J. D. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J. Biol. Chem. 279, 4737–4742 (2004).
Diaz, B. et al. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci. Signal. 2, ra53 (2009).
Burnham, D. N., Uhlinger, D. J. & Lambeth, J. D. Diradylglycerol synergizes with an anionic amphiphile to activate superoxide generation and phosphorylation of p47phox in a cell-free system from human neutrophils. J. Biol. Chem. 265, 17550–17559 (1990).
Nauseef, W. M., McCormick, S., Renee, J., Leidal, K. G. & Clark, R. A. Functional domain in an arginine-rich carboxyl-terminal region of p47phox. J. Biol. Chem. 268, 23646–23651 (1993).
Leto, T. L., Morand, S., Hurt, D. & Ueyama, T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid. Redox Signal 11, 2607–2619 (2009).
Martyn, K. D., Frederick, L. M., von Loehneysen, K., Dinauer, M. C. & Knaus, U. G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal. 18, 69–82 (2006).
Banfi, B., Clark, R. A., Steger, K. & Krause, K. H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 278, 3510–3513 (2003).
Brandes, R. P. & Schroder, K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovasc. Med. 18, 15–19 (2008).
Brandes, R. P., Weissmann, N. & Schroder, K. NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med. 49, 687–706 (2010).
Lassegue, B. & Griendling, K. K. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb. Vasc. Biol. 30, 653–661 (2010).
Selemidis, S., Sobey, C. G., Wingler, K., Schmidt, H. H. & Drummond, G. R. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol. Ther. 120, 254–291 (2008).
Lyle, A. N. et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 105, 249–259 (2009).
Morand, S. et al. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 23, 1205–1218 (2009).
Block, K. et al. NAD(P)H oxidases regulate HIF-2α protein expression. J. Biol. Chem. 282, 8019–8026 (2007).
Brar, S. S. et al. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am. J. Physiol. Cell Physiol. 285, C353–C369 (2003).
Brar, S. S. et al. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am. J. Physiol. Cell Physiol. 282, C1212–C1224 (2002).
Choi, J. A. et al. Pro-survival of estrogen receptor-negative breast cancer cells is regulated by a BLT2-reactive oxygen species-linked signaling pathway. Carcinogenesis 31, 543–551 (2010).
Huang, H. S., Liu, Z. M., Chen, P. C., Tseng, H. Y. & Yeh, B. W. TG-interacting factor-induced superoxide production from NADPH oxidase contributes to the migration/invasion of urothelial carcinoma. Free Radic. Biol Med. (in the press).
Huang, W. C., Li, X., Liu, J., Lin, J. & Chung, L. W. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol. Cancer Res. 10, 133–142 (2012).
Juhasz, A. et al. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radic. Res. 43, 523–532 (2009). mRNA expression levels of NOX catalytic and regulatory subunits are presented in a large variety of cultured cancer cell lines and tissues and are compared with normal controls.
Kumar, B., Koul, S., Khandrika, L., Meacham, R. B. & Koul, H. K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 68, 1777–1785 (2008).
Prata, C. et al. Nox-generated ROS modulate glucose uptake in a leukaemic cell line. Free Radic. Res. 42, 405–414 (2008).
Seals, D. F. et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell 7, 155–165 (2005).
Shimada, K., Fujii, T., Anai, S., Fujimoto, K. & Konishi, N. ROS generation via NOX4 and its utility in the cytological diagnosis of urothelial carcinoma of the urinary bladder. BMC Urol. 11, 22 (2011).
Vaquero, E. C., Edderkaoui, M., Pandol, S. J., Gukovsky, I. & Gukovskaya, A. S. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J. Biol. Chem. 279, 34643–34654 (2004). This paper describes the first evidence that NOX4-derived ROS mediates cell survival in cancer cells.
Yamaura, M. et al. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 69, 2647–2654 (2009).
Arnold, R. S. et al. Nox1 expression determines cellular reactive oxygen and modulates c-fos-induced growth factor, interleukin-8, and Cav-1. Am. J. Pathol. 171, 2021–2032 (2007).
Bauer, K. M., Hummon, A. B. & Buechler, S. Right-side and left-side colon cancer follow different pathways to relapse. Mol. Carcinog. 51, 411–421 (2012).
Block, K. et al. The NADPH oxidase subunit p22phox inhibits the function of the tumor suppressor protein tuberin. Am. J. Pathol. 176, 2447–2455 (2010).
Desouki, M. M., Kulawiec, M., Bansal, S., Das, G. M. & Singh, K. K. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol. Ther. 4, 1367–1373 (2005). This paper demonstrates the existence of crosstalk between the mitochondria and NADPH oxidase in cancer.
Fritz, G., Just, I. & Kaina, B. Rho GTPases are over-expressed in human tumors. Int. J. Cancer 81, 682–687 (1999).
Fukuyama, M. et al. Overexpression of a novel superoxide-producing enzyme, NADPH oxidase 1, in adenoma and well differentiated adenocarcinoma of the human colon. Cancer Lett. 221, 97–104 (2005).
Gomez del Pulgar, T., Benitah, S. A., Valeron, P. F., Espina, C. & Lacal, J. C. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays 27, 602–613 (2005).
Hoffmann, M. et al. A functional polymorphism in the NAD(P)H oxidase subunit CYBA is related to gene expression, enzyme activity, and outcome in non-Hodgkin lymphoma. Cancer Res. 70, 2328–2338 (2010).
Kamiguti, A. S. et al. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J. Immunol. 175, 8424–8430 (2005).
Lan, Q. et al. Genetic polymorphisms in the oxidative stress pathway and susceptibility to non-Hodgkin lymphoma. Hum. Genet. 121, 161–168 (2007).
Lim, S. D. et al. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 62, 200–207 (2005).
Shimada, K. et al. A novel human AlkB homologue, ALKBH8, contributes to human bladder cancer progression. Cancer Res. 69, 3157–3164 (2009).
Weyemi, U. et al. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr. Relat. Cancer 17, 27–37 (2010).
Xia, C. et al. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 67, 10823–10830 (2007).
Durak, I., Canbolat, O., Kavutcu, M., Ozturk, H. S. & Yurtarslani, Z. Activities of total, cytoplasmic, and mitochondrial superoxide dismutase enzymes in sera and pleural fluids from patients with lung cancer. J. Clin. Lab. Anal. 10, 17–20 (1996).
Bostwick, D. G. et al. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer 89, 123–134 (2000).
Curtis, C. D., Thorngren, D. L. & Nardulli, A. M. Immunohistochemical analysis of oxidative stress and DNA repair proteins in normal mammary and breast cancer tissues. BMC Cancer 10, 9 (2010).
Evans, P. & Halliwell, B. Free radicals and hearing. Cause, consequence, and criteria. Ann. NY Acad. Sci. 884, 19–40 (1999).
Novo, E. & Parola, M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair 1, 5 (2008).
Kartha, G. K. et al. Renal mitochondrial damage and protein modification in type-2 diabetes. Acta Diabetol. 45, 75–81 (2008).
Spencer, N. Y. et al. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J. Biol. Chem. 286, 8977–8987 (2011).
Weyemi, U. et al. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene 31, 1117–1129 (2012).
Burdon, R. H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic. Biol. Med. 18, 775–794 (1995).
Burdon, R. H., Gill, V. & Rice-Evans, C. Oxidative stress and tumour cell proliferation. Free Radic. Res. Commun. 11, 65–76 (1990).
Burdon, R. H. & Rice-Evans, C. Free radicals and the regulation of mammalian cell proliferation. Free Radic. Res. Commun. 6, 345–358 (1989).
Bae, Y. S. et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 217–221 (1997).
Mahadev, K. et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 24, 1844–1854 (2004).
Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K. & Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 (1995).
Chen, K., Kirber, M. T., Xiao, H., Yang, Y. & Keaney, J. F. Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 181, 1129–1139 (2008).
Lyle, A. N. & Griendling, K. K. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology 21, 269–280 (2006).
Mesquita, F. S. et al. Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol. Reprod. 82, 341–351 (2010).
Wagner, B. et al. Mitogenic signaling via platelet-derived growth factor β in metanephric mesenchymal cells. J. Am. Soc. Nephrol. 18, 2903–2911 (2007).
Reddy, M. M. et al. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia 25, 281–289 (2011).
Sastry, S. K. & Elferink, L. A. Checks and balances: interplay of RTKs and PTPs in cancer progression. Biochem. Pharmacol. 82, 435–440 (2011).
Rudolph, T. K. & Freeman, B. A. Transduction of redox signaling by electrophile-protein reactions. Sci. Signal. 2, re7 (2009).
Chiarugi, P. & Cirri, P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem. Sci. 28, 509–514 (2003).
Lee, J. K. et al. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology 133, 1637–1648 (2007).
Lee, S. R., Kwon, K. S., Kim, S. R. & Rhee, S. G. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 273, 15366–15372 (1998).
Woo, H. A. et al. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science 300, 653–656 (2003).
Lou, Y. W. et al. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS J. 275, 69–88 (2008).
Groen, A. et al. Differential oxidation of protein-tyrosine phosphatases. J. Biol. Chem. 280, 10298–10304 (2005).
Rhee, S. G., Chang, T. S., Bae, Y. S., Lee, S. R. & Kang, S. W. Cellular regulation by hydrogen peroxide. J. Am. Soc. Nephrol. 14, S211–S215 (2003).
Wright, V. P., Reiser, P. J. & Clanton, T. L. Redox modulation of global phosphatase activity and protein phosphorylation in intact skeletal muscle. J. Physiol. 587, 5767–5781 (2009).
Chan, E. C., Jiang, F., Peshavariya, H. M. & Dusting, G. J. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol. Ther. 122, 97–108 (2009).
Ushio-Fukai, M. Localizing NADPH oxidase-derived ROS. Sci. STKE 2006, re8 (2006).
Almo, S. C. et al. Structural genomics of protein phosphatases. J. Struct. Funct. Genomics 8, 121–140 (2007).
Sancho, P. & Fabregat, I. NADPH oxidase NOX1 controls autocrine growth of liver tumor cells through up-regulation of the epidermal growth factor receptor pathway. J. Biol. Chem. 285, 24815–24824 (2010).
Nitsche, C. et al. The phosphatase PHLPP1 regulates Akt2, promotes pancreatic cancer cell death, and inhibits tumor formation. Gastroenterology 142, 377–387.e5 (2012).
Naughton, R., Quiney, C., Turner, S. D. & Cotter, T. G. Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia 23, 1432–1440 (2009).
Seo, J. M. et al. Up-regulation of BLT2 is critical for the survival of bladder cancer cells. Exp. Mol. Med. 43, 129–137 (2011).
Mochizuki, T. et al. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 25, 3699–3707 (2006).
Luo, J. et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 (2001).
Puca, R. et al. Nox1 is involved in p53 deacetylation and suppression of its transcriptional activity and apoptosis. Free Radic. Biol. Med. 48, 1338–1346 (2010). This is the first example that NOX1 activates SIRT1 deacetylase, which inhibits p53-dependent cell apoptosis.
Vaziri, H. et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001).
Olmos, Y., Brosens, J. J. & Lam, E. W. Interplay between SIRT proteins and tumour suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resist. Updat. 14, 35–44 (2011).
Frescas, D., Valenti, L. & Accili, D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 280, 20589–20595 (2005).
Laurent, E. et al. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int. J. Cancer 123, 100–107 (2008).
Heo, J. Redox control of GTPases: from molecular mechanisms to functional significance in health and disease. Antioxid. Redox Signal. 14, 689–724 (2011).
Lander, H. M. Ogiste, J.S., Teng, K.K. & Novogrodsky, A. p21ras as a common signaling target of reactive free radicals and cellular redox stress. J. Biol. Chem. 270, 21195–21198 (1995).
Adachi, Y. et al. Oncogenic Ras upregulates NADPH oxidase 1 gene expression through MEK-ERK-dependent phosphorylation of GATA-6. Oncogene 27, 4921–4932 (2008).
Mitsushita, J., Lambeth, J. D. & Kamata, T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 64, 3580–3585 (2004). This paper describes the cooperation of RAS and NOX1 in cellular transformation.
Kajla, S. et al. A crucial role for Nox 1 in redox-dependent regulation of Wnt-β-catenin signaling. FASEB J. 26, 2049–2059 (2012).
Rimerman, R. A., Gellert-Randleman, A. & Diehl, J. A. Wnt1 and MEK1 cooperate to promote cyclin D1 accumulation and cellular transformation. J. Biol. Chem. 275, 14736–14742 (2000).
Du, J. et al. Regulation of pancreatic cancer growth by superoxide. Mol. Carcinog. 5 Mar 2012 (doi:10.1002/mc.21891).
Cantley, L. C. The phosphoinositide 3-kinase pathway. Science 296, 1655–1657 (2002).
Luo, J., Manning, B. D. & Cantley, L. C. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 4, 257–262 (2003).
Manning, B. D. & Cantley, L. C. AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 (2007).
Pópulo, H., Lopes, J. M. & Soares, P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 13, 1886–1918 (2012).
Porta, C. & Figlin, R. A. Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney cancer, and the therapeutic potential of phosphatidylinositol-3-kinase/Akt inhibitors. J. Urol. 182, 2569–2577 (2009).
Silvera, D., Formenti, S. C. & Schneider, R. J. Translational control in cancer. Nature Rev. Cancer 10, 254–266 (2010).
Lee, S. R. et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 277, 20336–20342 (2002).
Coant, N. et al. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol. Cell. Biol. 30, 2636–2650 (2010).
Cui, W. et al. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology 54, 949–958 (2011).
Kwon, J. et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl Acad. Sci. USA 101, 16419–16424 (2004).
Nisbet, R. E. et al. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am. J. Respir. Cell Mol. Biol. 42, 482–490 (2010).
Gan, X., Wang, J., Su, B. & Wu, D. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 286, 10998–11002 (2011).
Lamouille, S., Connolly, E., Smyth, J. W., Akhurst, R. J. & Derynck, R. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J. Cell Sci. 125, 1259–1273 (2012).
Zinzalla, V., Stracka, D., Oppliger, W. & Hall, M. N. Activation of mTORC2 by association with the ribosome. Cell 144, 757–768 (2011).
Plas, D. R. & Thompson, C. B. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J. Biol. Chem. 278, 12361–12366 (2003).
Sonenberg, N. Translation factors as effectors of cell growth and tumorigenesis. Curr. Opin. Cell Biol. 5, 955–960 (1993).
Clifford, S. C. et al. The pVHL-associated SCF ubiquitin ligase complex: molecular genetic analysis of elongin B and C, Rbx1 and HIF-1α in renal cell carcinoma. Oncogene 20, 5067–5074 (2001).
Kaelin, W. G. Jr. The von hippel-lindau tumor suppressor protein: an update. Methods Enzymol. 435, 371–383 (2007).
Pause, A. et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc. Natl Acad. Sci. USA 94, 2156–2161 (1997).
Min, J. H. et al. Structure of an HIF-1α -pVHL complex: hydroxyproline recognition in signaling. Science 296, 1886–1889 (2002).
Maranchie, J. K. & Zhan, Y. Nox4 is critical for hypoxia-inducible factor 2-α transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res. 65, 9190–9193 (2005).
Isaacs, J. S. et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8, 143–153 (2005).
Sudarshan, S. et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1α stabilization by glucose-dependent generation of reactive oxygen species. Mol. Cell. Biol. 29, 4080–4090 (2009).
Maraldi, T. et al. VEGF-induced ROS generation from NAD(P)H oxidases protects human leukemic cells from apoptosis. Int. J. Oncol. 36, 1581–1589 (2010).
Ushio-Fukai, M. & Nakamura, Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 266, 37–52 (2008).
Diebold, I., Petry, A., Hess, J. & Gorlach, A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell 21, 2087–2096 (2010). This is the first paper to show that hypoxia-inducible factors regulate NOX4 gene expression.
Diebold, I. et al. The HIF1 target gene NOX2 promotes angiogenesis through urotensin-II. J. Cell Sci. 125, 956–964 (2012).
Goyal, P. et al. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic. Biol. Med. 36, 1279–1288 (2004).
Tojo, T. et al. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111, 2347–2355 (2005).
Vallet, P. et al. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience 132, 233–238 (2005).
Arbiser, J. L. et al. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc. Natl Acad. Sci. USA 99, 715–720 (2002). This paper is the first evidence that NOX1 is involved in tumour angiogenesis in vitro and in vivo.
Govindarajan, B. et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J. Clin. Invest. 117, 719–729 (2007). This paper describes the cooperation of AKT and NOX4 in invasive melanoma cell growth.
Komatsu, D., Kato, M., Nakayama, J., Miyagawa, S. & Kamata, T. NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression. Oncogene 27, 4724–4732 (2008).
Garrido-Urbani, S. et al. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARα mediated mechanism. PLoS ONE 6, e14665 (2011).
Tobar, N., Guerrero, J., Smith, P. C. & Martinez, J. NOX4-dependent ROS production by stromal mammary cells modulates epithelial MCF-7 cell migration. Br. J. Cancer 103, 1040–1047 (2010).
Hou, Z., Falcone, D. J., Subbaramaiah, K. & Dannenberg, A. J. Macrophages induce COX-2 expression in breast cancer cells: role of IL-1β autoamplification. Carcinogenesis 32, 695–702 (2011).
Fitzgerald, J. P. et al. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PLoS ONE 7, e30712 (2012).
Corzo, C. A. et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701 (2009).
Courtneidge, S. A. Cell migration and invasion in human disease: the Tks adaptor proteins. Biochem. Soc. Trans. 40, 129–132 (2012).
Diaz, B. & Courtneidge, S. A. Redox signaling at invasive microdomains in cancer cells. Free Rad. Biol. Med. 52, 247–256 (2012). This review outlines redox signalling events mediated by invadopodia NADPH oxidase complexes and their contribution to cancer cell invasion.
Wang, Y. & McNiven, M. A. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J. Cell Biol. 196, 375–385 (2012).
Destaing, O., Block, M. R., Planus, E. & Albiges-Rizo, C. Invadosome regulation by adhesion signaling. Curr. Opin. Cell Biol. 23, 597–606 (2011).
Giannoni, E., Taddei, M. L. & Chiarugi, P. Src redox regulation: again in the front line. Free Radic. Biol. Med. 49, 516–527 (2010).
Guarino, M. Src signaling in cancer invasion. J. Cell. Physiol. 223, 14–26 (2010).
Yeatman, T. J. A renaissance for SRC. Nature Rev. Cancer 4, 470–480 (2004).
Gianni, D., Taulet, N., DerMardirossian, C. & Bokoch, G. M. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol. Biol. Cell 21, 4287–4298 (2010).
Baumer, A. T. et al. Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for α-platelet-derived growth factor receptor-induced production of reactive oxygen species. J. Biol. Chem. 283, 7864–7876 (2008).
Chowdhury, A. K. et al. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J. Biol. Chem. 280, 20700–20711 (2005).
Gianni, D., Bohl, B., Courtneidge, S. A. & Bokoch, G. M. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol. Biol. Cell 19, 2984–2994 (2008).
Gianni, D. et al. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci. Signal. 2, ra54 (2009).
Block, K. et al. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J. Biol. Chem. 283, 24061–24076 (2008).
Li, Q., Zhang, Y., Marden, J. J., Banfi, B. & Engelhardt, J. F. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem. J. 411, 531–541 (2008).
Binker, M. G., Binker-Cosen, A. A., Richards, D., Oliver, B. & Cosen-Binker, L. I. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem. Biophys. Res. Commun. 379, 445–450 (2009).
Steinbrenner, H. et al. Tumor promoter TPA stimulates MMP-9 secretion from human keratinocytes by activation of superoxide-producing NADPH oxidase. Free Radic. Res. 39, 245–253 (2005).
Yoon, S. O., Park, S. J., Yoon, S. Y., Yun, C. H. & Chung, A. S. Sustained production of H2O2 activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-κ B pathway. J. Biol. Chem. 277, 30271–30282 (2002).
Grote, K. et al. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ. Res. 92, e80–e86 (2003).
Seo, J. M., Park, S. & Kim, J. H. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J. Biol. Chem. 287, 13840–13849 (2012).
Choi, J. A., Kim, E. Y., Song, H., Kim, C. & Kim, J. H. Reactive oxygen species are generated through a BLT2-linked cascade in Ras-transformed cells. Free Radic. Biol. Med. 44, 624–634 (2008).
Kim, E. Y., Seo, J. M., Cho, K. J. & Kim, J. H. Ras-induced invasion and metastasis are regulated by a leukotriene B4 receptor BLT2-linked pathway. Oncogene 29, 1167–1178 (2010).
Kim, E. Y. et al. BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen species-linked pathway. Free Radic. Biol. Med. 49, 1072–1081 (2010).
Shinohara, M. et al. Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J. Biol. Chem. 285, 4481–4488 (2010).
Yang, J. Q. et al. v-Ha-RaS oncogene upregulates the 92-kDa type IV collagenase (MMP-9) gene by increasing cellular superoxide production and activating NF-κB. Free Radic. Biol. Med. 31, 520–529 (2001).
Acknowledgements
The authors acknowledge H. E. Abboud, R. A. Clark and R. Li for critical reading of the Review and F. Wauquier for unconditional assistance. Supported by Veterans Administration Career Development Award, NIH R01 NCI CA131272 (K.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Focal adhesions
-
Specific types of large macromolecular assemblies through which both mechanical force and regulatory signals are transmitted.
- Invadopodia
-
Protrusions of the plasma membrane that contain adhesive proteins and proteolytic enzymes.
- Dismutation
-
A specific type of redox reaction in which a species is simultaneously reduced and oxidized to form two different products, in this case molecular oxygen and hydrogen peroxide.
Rights and permissions
About this article
Cite this article
Block, K., Gorin, Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer 12, 627–637 (2012). https://doi.org/10.1038/nrc3339
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3339
This article is cited by
-
Targeting ROS production through inhibition of NADPH oxidases
Nature Chemical Biology (2023)
-
The pentose phosphate pathway in health and disease
Nature Metabolism (2023)
-
The role of hypoxia on prostate cancer progression and metastasis
Molecular Biology Reports (2023)
-
A maternal high-fat diet induces fetal origins of NASH-HCC in mice
Scientific Reports (2022)