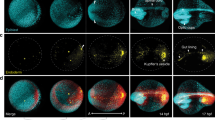
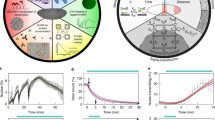
Abstract
This protocol describes imaging and computational tools to collect and analyze live imaging data of embryonic cell migration. Our five-step protocol requires a few weeks to move through embryo preparation and four-dimensional (4D) live imaging using multi-photon microscopy, to 3D cell tracking using image processing, registration of tracking data and their quantitative analysis using computational tools. It uses commercially available equipment and requires expertise in microscopy and programming that is appropriate for a biology laboratory. Custom-made scripts are provided, as well as sample datasets to permit readers without experimental data to carry out the analysis. The protocol has offered new insights into the genetic control of cell migration during Drosophila gastrulation. With simple modifications, this systematic analysis could be applied to any developing system to define cell positions in accordance with the body plan, to decompose complex 3D movements and to quantify the collective nature of cell migration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
McMahon, A., Supatto, W., Fraser, S.E. & Stathopoulos, A. Dynamic analyses of Drosophila Gastrulation provide insights into collective cell migration. Science 322, 1546–1550 (2008).
Keller, P.J., Schmidt, A.D., Wittbrodt, J. & Stelzer, E.H.K. Reconstruction of Zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069 (2008).
Leptin, M. & Grunewald, B. Cell shape changes during gastrulation in Drosophila . Development 110, 73–84 (1990).
Kam, Z., Minden, J.S., Agard, D.A., Sedat, J.W. & Leptin, M. Drosophila gastrulation: analysis of cell shape changes in living embryos by three-dimensional fluorescence microscopy. Development 112, 365–370 (1991).
Irvine, K.D. & Wieschaus, E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development 120, 827–841 (1994).
Bertet, C., Sulak, L. & Lecuit, T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667–671 (2004).
Smallhorn, M., Murray, M.J. & Saint, R. The epithelial-mesenchymal transition of the Drosophila mesoderm requires the Rho GTP exchange factor Pebble. Development 131, 2641–2651 (2004).
Debarre, D. et al. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat. Methods 3, 47–53 (2006).
Welte, M.A., Gross, S.P., Postner, M., Block, S.M. & Wieschaus, E.F. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell 92, 547–557 (1998).
Hartenstein, V. Atlas of Drosophila development. In The Development of Drosophila melanogaster (eds. Bate, M. & Martinez Arias, A.) 36 and 52 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA, 1993).
Supatto, W., Fraser, S.E. & Vermot, J. An all-optical approach for probing microscopic flows in living embryos. Biophys. J. 95, L29–L31 (2008).
Supatto, W. et al. In vivo modulation of morphogenetic movements in Drosophila embryos with femtosecond laser pulses. Proc. Natl. Acad. Sci. USA 102, 1047–1052 (2005).
Hopt, A. & Neher, E. Highly nonlinear photodamage in two-photon fluorescence microscopy. Biophys. J. 80, 2029–2036 (2001).
Ji, N., Magee, J.C. & Betzig, E. High-speed, low-photodamage nonlinear imaging using passive pulse splitters. Nat. Methods 5, 197–202 (2008).
Beaurepaire, E. & Mertz, J. Epifluorescence collection in two-photon microscopy. Appl. Optics 41, 5376–5382 (2002).
Vermot, J., Fraser, S.E. & Liebling, M. Fast fluorescence microscopy for imaging the dynamics of embryonic development. HFSP J. 2, 143–155 (2008).
Debarre, D. et al. Velocimetric third-harmonic generation microscopy: micrometer-scale quantification of morphogenetic movements in unstained embryos. Opt. Lett. 29, 2881–2883 (2004).
Zamir, E.A., Czirok, A., Cui, C., Little, C.D. & Rongish, B.J. Mesodermal cell displacements during avian gastrulation are due to both individual cell-autonomous and convective tissue movements. Proc. Natl. Acad. Sci. USA 103, 19806–19811 (2006).
Liebling, M., Forouhar, A.S., Gharib, M., Fraser, S.E. & Dickinson, M.E. Four-dimensional cardiac imaging in living embryos via postacquisition synchronization of nongated slice sequences. J. Biomed. Opt. 10, 054001 (2005).
Tyszka, J.M., Ewald, A.J., Wallingford, J.B. & Fraser, S.E. New tools for visualization and analysis of morphogenesis in spherical embryos. Dev. Dyn. 234, 974–983 (2005).
Guan, J.L. Cell Migration: Developmental Methods and Protocols (Humana Press, Totowa, NJ, USA, 2005).
Greenspan, R.J. Fly Pushing: The Theory and Practice of Drosophila Genetics. 17–21 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA, 2004).
Reed, B., McMillan, S. & Chaudhary, R. The Preparation of Drosophila embryos for live-imaging using the hanging drop protocol. J. Vis. Exp. 25 (2009).
Oheim, M., Beaurepaire, E., Chaigneau, E., Mertz, J. & Charpak, S. Two-photon microscopy in brain tissue: parameters influencing the imaging depth. J. Neurosci. Methods 111, 29–37 (2001).
Acknowledgements
We thank M. Liebling for advice on Imaris and Matlab, and the Caltech Biological Imaging Center for sharing equipment. This work was supported by grants to AS from National Institutes of Health (R01 GM078542), the Searle Scholars Program and the March of Dimes (Basil O'Conner Starter Scholar Award, 5-FY06-145), grants to SF from the Caltech Beckman Institute and National Institutes of Health (Center for Excellence in Genomic Science grant P50HG004071), and fellowship to W.S. from the Caltech Biology Division.
Author information
Authors and Affiliations
Contributions
W.S. designed the experimental workflow and the computational analysis. W.S. and A.M. acquired and processed the data. W.S., A.M., S.E.F. and A.S. wrote the paper.
Note: Supplementary information is available via the HTML version of this article.
Corresponding authors
Supplementary information
Supplementary Data 1. Matlab scripts.
See Table 2 for details. (ZIP 15 kb)
Supplementary Data 2. Sample datasets.
Mesoderm, ectoderm, and midline tracking sample datasets are included. (ZIP 801 kb)
Supplementary Movie 1.
3D image of an entire klarsicht;nls-GFP embryo at the onset of mesoderm cell spreading. This movie shows the good signal obtained from the mesoderm furrow. The nls-GFP signal is diffuse during cell division (see the anterior pole). This dataset has been acquired in slightly different conditions compared to the rest of this protocol to image the embryo with a larger field of view using a 20x 0.95NA objective from Olympus. The nls-GFP Drosophila embryo (Bloomington Stock Center, stock number 5623) has been imaged on a TriMScope 2PEF setup provided LaVision BioTech company. (MOV 2754 kb)
Supplementary Movie 2.
3D schematic animation of quantitative imaging of Drosophila embryos. This movie successively shows (i) the Drosophila embryo shape and body plan with the position of the midline; (ii) the position of the image acquisition field of view on the ventral side of the embryo; (iii) the position and shape of the ectoderm and mesoderm layers at the onset of mesoderm spreading, with a representation of the ventral furrow; (iv) a 3D view of the cylindrical coordinate system adapted to the shape of the embryo in the imaging area; (v) a 3D representation of morphogenetic movements showing the ectoderm convergence-extension during germband extension (GBE), the mesoderm furrow collapse and the mesoderm spreading. (MOV 6389 kb)
Supplementary Movie 3.
Example of angular drift during image acquisition. View of the ectoderm layer from the ventral side in H2A-GFP expressing embryos. Arrow indicates the position of midline cells and highlights the angular drift. It corresponds to the drift corrected in Fig. 5. The specific shape of the nuclei from midline cells (elongated along the anterior-posterior axis) is clear. Anterior-posterior axis is vertical, posterior is up. (MOV 3432 kb)
Rights and permissions
About this article
Cite this article
Supatto, W., McMahon, A., Fraser, S. et al. Quantitative imaging of collective cell migration during Drosophila gastrulation: multiphoton microscopy and computational analysis. Nat Protoc 4, 1397–1412 (2009). https://doi.org/10.1038/nprot.2009.130
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.130
This article is cited by
-
Multiphoton intravital microscopy of rodents
Nature Reviews Methods Primers (2022)
-
Biologically constrained optimization based cell membrane segmentation in C. elegans embryos
BMC Bioinformatics (2017)
-
A workflow to process 3D+time microscopy images of developing organisms and reconstruct their cell lineage
Nature Communications (2016)
-
Dynamic imaging of the growth plate cartilage reveals multiple contributors to skeletal morphogenesis
Nature Communications (2015)
-
Advances in whole-embryo imaging: a quantitative transition is underway
Nature Reviews Molecular Cell Biology (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.